
Niobium, the lightest refractory metal, possesses a series of outstanding properties including high melting point, high specific strength, low vapor pressure, excellent cold workability, high chemical stability, and strong resistance to acid and alkali corrosion. It is widely used in mechanical manufacturing, aerospace, nuclear industry, and medical fields, such as aerospace engines and nuclear reactor structural materials. In recent years, China's aerospace technology advancement has driven heightened demands for high-temperature materials. Numerous structural components in high thrust-to-weight ratio engines and hypersonic vehicles require maintaining high strength above 1300°C. Niobium alloys, characterized by low density, high melting point, and superior high-temperature performance, are extensively employed in manufacturing critical high-temperature structural parts for rockets, spacecraft, and hypersonic aircraft. However, increasingly stringent operating conditions and complex geometries pose significant challenges to conventional niobium alloys and their forming methods. Additive manufacturing has emerged as the preferred solution for producing complex-shaped niobium alloy components capable of withstanding extreme temperatures, with the preparation of alloy powders for additive manufacturing being a critical technology [1]. Currently, production equipment and core technologies for spherical refractory alloy powders used in additive manufacturing are primarily controlled by countries such as Germany, the United States, and Japan. Due to the high difficulty of preparation and stringent performance requirements, few research institutions are engaged in this field, representing a weakness in China's refractory metal additive manufacturing. Particularly, the development of high-quality spherical metal powders for laser-based additive manufacturing lags behind developed nations, failing to meet the urgent demands of China's high-end manufacturing sectors like aerospace and nuclear power. Therefore, there is an urgent need to conduct innovative research encompassing fundamental frontiers, key technologies, and industrialization for high-quality spherical refractory metal powders used in additive manufacturing. This will support the development of intelligent manufacturing technologies in China's aviation, aerospace, nuclear power, and other sectors.
Common techniques for preparing spherical refractory metal powders include the plasma rotating electrode method and radiofrequency induction plasma spheroidization. The rotating electrode spheroidization method is generally used for preparing powders of titanium alloys, magnesium alloys, and high-temperature alloys [2-3]. In contrast, the radiofrequency induction plasma spheroidization method features a high plasma torch temperature, enabling the processing of refractory metal powders. It is an effective means for obtaining spherical niobium alloy powders with high density, good sphericity, and high purity, offering significant advantages over other processes. In the RF-induced plasma spheroidization process, parameters such as plasma power, feed rate, and gas flow rate are critical factors for enhancing powder spheroidization efficiency. Zhou Xiaobin et al. [4] and Gu Zhongtao et al. [5] respectively investigated the plasma spheroidization processes for producing molybdenum and tungsten powders. Their results indicate that optimal power control is crucial for enhancing powder spheroidization rates, while reducing feed rates and carrier gas flow rates improves powder sphericity. Additionally, studies on induction plasma spheroidization of niobium alloys have been conducted [6-7]. Currently, while there are successful cases of preparing spherical refractory metals and alloys via induction plasma spheroidization [8-12], relying solely on experimental trial-and-error to determine empirical parameters is not only time-consuming and labor-intensive but also highly costly. Theoretical calculations or simulation modeling have become essential tools for studying microstructural evolution under extreme conditions. These related studies [13-21] have significantly facilitated the manufacturing of spheroidization equipment and the optimization of spheroidization processes.
Researchers worldwide have successfully produced spherical refractory metal powders such as W, Mo, Ta, and Nb using induction plasma technology. During induction plasma spheroidization, the extremely high temperatures within the plasma torch necessitate consideration of alloy element vaporization losses. Powder particles melt into droplets under the influence of high-temperature plasma. During their descent, velocity differences between the droplets and internal bubbles create favorable kinetic conditions for bubble expulsion. However, existing research on induction plasma spheroidization primarily focuses on the influence of process parameters on spheroidization effectiveness, with limited attention given to the vaporization loss of alloying elements and the removal of impurities such as O and N during the process. Therefore, this study aims to provide a theoretical and experimental foundation for reducing the vaporization loss of alloying elements and promoting the removal of impurities like O and N during the spheroidization of niobium alloys.
1. Experiment
Before and after plasma spheroidization of Nb alloys, both the total powder mass and the content of certain elements undergo changes. The primary causes of these elemental content variations include the volatilization of surface elements from molten metal droplets and the removal of partially gaseous elements after bubble formation. Therefore, this study investigates the fundamental mechanisms governing Nb element loss and O/N bubble removal during plasma spheroidization of Nb alloys. Key aspects include the effects of temperature and particle size on Nb loss and bubble removal. Based on these findings, experimental studies were conducted in conjunction with the spheroidization production process.
1.1 Theoretical Calculation Methods
Theoretical calculations were conducted to investigate the effects of temperature and powder particle size on alloy element vaporization losses and bubble removal. Parameters such as velocity, temperature, forces, heat flux, and phase state during particle descent vary with drop height, necessitating numerical methods to solve corresponding differential equations. In this study, the RK4 algorithm was primarily applied to solve these differential equations. The following outlines the relevant theoretical research methods.
1) Vaporization Loss of Alloying Elements
During plasma spheroidization, the plasma torch region reaches extremely high temperatures, with the body torch and its extended high-temperature zone averaging above 3000°C. Metal particles melt into droplets within this zone. Although the residence time of metal droplets is brief, vaporization loss is unavoidable at such elevated temperatures. The length of the high-temperature zone is determined based on the TekSphero-80kW induction plasma spheroidizing equipment manufactured by Tekna, with a high-temperature zone length of 0.25m. The temperature selected for calculations is the average temperature of the high-temperature zone. Element vaporization losses primarily occur during the phase transition from solid to liquid, specifically during the period after particle melting and before solidification. During flight, metal particles primarily acquire heat through convective heat transfer and environmental radiation. The heat exchanged per unit time Φ (in W) can be expressed as [22-23]:
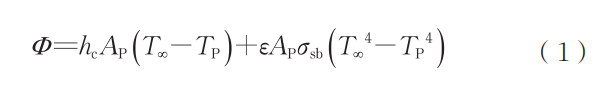
where:
T∞ is the ambient temperature, K; TP is the gas-particle temperature, K; AP is the particle surface area, m²; hc is the particle surface heat transfer coefficient, W/(K·m²). The heat transfer coefficient is primarily influenced by temperature, material composition, and the relative velocity between the particle and the gas flow. The convective heat transfer coefficients of particles at different temperatures used in the calculation are shown in Table 1; ε is the emissivity; σsb is the Stefan-Boltzmann constant, 5.67×10⁻⁸ W/(m²·K⁴). The atmosphere for particle spheroidization is argon. For monoatomic molecules like argon or symmetric diatomic molecules like hydrogen, the gas exhibits weak heat absorption and radiation capabilities. Convective heat transfer is the primary heat exchange mechanism between the gas and particles, allowing the radiation heat transfer term to be neglected in calculations.

During particle heating and cooling, the heat exchange between the particle and its environment, along with the particle temperature change, follows the following differential equation [22-23]:
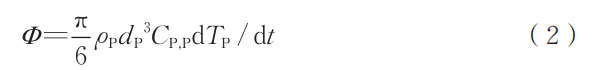
where ρP is the particle density, kg/m³; dP is the particle diameter, m; CP and P are the particle specific heat capacities, J/(kg·K). During melting, the particle temperature remains constant, and the total heat absorption can be expressed as:
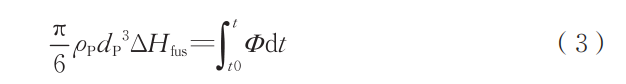
where AHUS is the enthalpy of fusion of the metal, J/kg. For this study, the melting point of Nb (2470°C) is used as the particle melting point. By combining Equations (1) to (3), numerical calculations can determine the duration of the phase between particle melting and solidification. This process is primarily influenced by factors such as particle material, particle size, and ambient temperature. After determining the droplet's sustained existence time, the loss rate of various elements at the droplet surface can be expressed as:
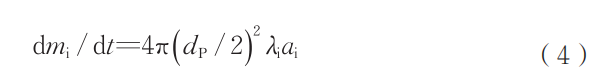
where mi is the mass of element i, kg; λi is the vaporization rate coefficient of element i per unit time and area, kg/(m²·s). The vaporization rate coefficient is primarily influenced by temperature and material composition. The surface vaporization rate λNb of Nb at different temperatures used in the calculation is shown in Table 2; ai denotes the activity of element i on the droplet surface. In Nb alloys, Nb serves as the matrix material, thus its activity value is set to 1 in calculations. The temperature difference between the ambient environment and the droplet surface significantly impacts λi, consequently affecting the element depletion rate. Solving the above differential equation enables calculation of the mass lost through vaporization of elements on the droplet surface, thereby determining the vaporization depletion rate.
2) Bubble Removal
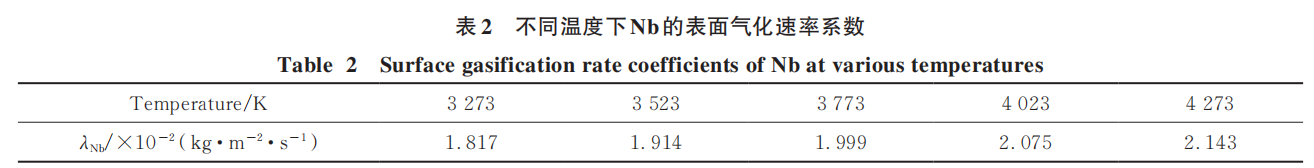
Typically, niobium alloy powder is produced by hydrogenating and comminuting niobium alloy ingots. Consequently, the feedstock powder used in induction plasma spheroidization is irregularly shaped. The relative density of green compacts obtained via powder metallurgy processes is generally around 95%. indicating the presence of relatively dispersed pores at the microscopic level. Gaseous elements within these pores may condense into bubbles when the powder melts into small droplets. Simultaneously, during melting and spheroidization, some surface-adsorbed gases may be entrained into the droplet interior due to droplet deformation. Gaseous elements such as oxygen and nitrogen within the particles may also precipitate and aggregate into bubbles within the high-temperature droplets. These phenomena collectively constitute the sources of bubbles within the droplets. During particle descent, rapid melting forms droplets. Before complete melting, bubbles cannot be expelled. After full melting, velocity differences between the droplet and trapped bubbles cause bubbles to be ejected through relative motion. The force analysis for this process is illustrated in Figure 1.
To simplify the model, it is assumed that the bubble is located at the center of the particle. The duration of the droplet state is calculated using the method introduced in the previous subsection. Due to the complex forces acting on the bubble, within this motion system, the bubble experiences gravitational force (mg), buoyancy force (F_b), and viscous drag force (F'ₚ-b). Its acceleration (a_b) is expressed according to Newton's laws of motion as follows:
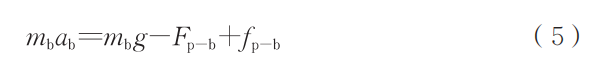
All forces in the equation are in units of N. The droplet experiences gravitational force (mpg), air resistance (fp-∞), the reaction force of bubble buoyancy (F'ₚ-b), and the reaction force of bubble viscous drag (f'ₚ-b). Its acceleration (ap) is expressed according to Newton's laws of motion as follows:
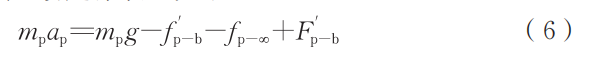
Since action and reaction forces are numerically equal, combining Equations (5) and (6) yields the acceleration expressions for the droplet and bubble:
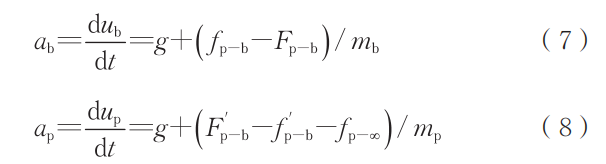
where ab is the bubble acceleration, m/s²; ab is the bubble acceleration, m/s². From these, the numerical difference formulas for solving particle velocity and bubble velocity can be further derived:
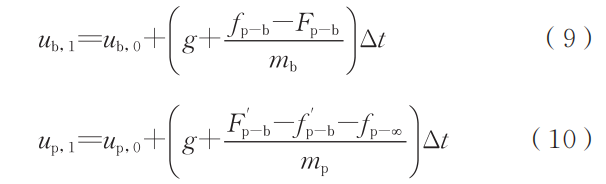
where ub,0 and ub,1 denote the bubble velocity before and after each iteration, respectively, in m/s; up,0 and up,1 denote the bubble velocity before and after each iteration, respectively, in m/s. Using these two equations, the change in relative position between the droplet and bubble during descent can be calculated, thereby determining the time required for the bubble to be expelled from the droplet. In a non-inertial reference frame, this calculation process can be simplified. In practice, due to the minute size of the droplet, air resistance cannot be neglected, placing it in a state of partial weightlessness. In a non-inertial frame, the bubble's weightlessness acceleration can be expressed as:
Substituting the various forces yields:
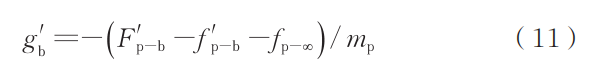
Considering that action and reaction forces are equal in magnitude and opposite in direction, the buoyant force acting on the bubble can be expressed as:
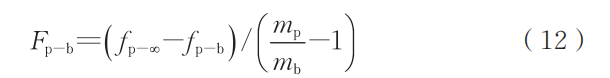
In the non-inertial frame, the droplet can be considered stationary, with the bubble overflowing from the droplet interior under the buoyancy force. During calculations, the air resistance on the droplet and the viscous resistance on the bubble can be determined using Newton's viscosity law. These forces are primarily influenced by factors such as gas flow velocity, gas viscosity, droplet diameter, droplet density, droplet viscosity, bubble diameter, and bubble density.
1.2 Experimental Research Method
Experiments investigated the effects of different processes on alloy element loss rates and gas-phase removal rates during spheroidization. Tests were conducted using a high-frequency induction plasma spheroidization system manufactured by Tekna, model TekSphero-80kW. The electrode-free induction plasma generator avoids powder contamination, making it particularly suitable for producing high-purity powders or materials. Additionally, it imposes virtually no restrictions on raw material melting temperatures. The experimental powder selected was Nb521 alloy powder (composition: W: 4.5%–5.5%, Mo: 1.5%–2.5%, Zr: 0.7%–1.5%, remainder Nb). Typically, spherical Nb alloy powders for additive manufacturing have a particle size of 15–53 μm. while the powder selected for this study has a particle size of 30–50 μm, as shown in the SEM image (Figure 2). After alloy elements vaporize in the high-temperature zone, they condense into finer powder particles in the low-temperature zone. Typically, these recondensed particles are extremely fine, with diameters below 5 μm. Some adhere to the inner walls of the spheroidization chamber, some move with the gas flow and separate from the gas at the filtration device, and others attach to the surfaces of the spheroidized particles. After spheroidization, the powder undergoes ultrasonic cleaning and sieving to remove fine particles adhering to the surface. The mass of the spheroidized powder, m₁, is then weighed. The mass of the powder before spheroidization, m₀, is also weighed beforehand. The alloy loss rate is calculated as 1 - m₁/m₀. The removal rate of gaseous elements is calculated by measuring the content of elements such as O and N before and after processing, then determining the removal rate based on the change in these elemental contents.
2 Results and Discussion
2.1 Vaporization Loss of Alloying Elements
Theoretical calculations were performed to determine the loss rate of Nb during spheroidization (considering that Nb is the primary element in niobium alloys), primarily investigating the effects of plasma torch average temperature and particle diameter on Nb loss. The gas flow rate, temperature zone length, and average temperature used in the calculations were based on the TekSphero-80kW induction plasma spheroidization equipment manufactured by Tekna, incorporating multiple references on numerical simulations of the induction plasma spheroidization process by HOSSAIN, TONG, NAM, et al. [13-17]. The average gas flow velocity in the spheroidization chamber is 1.8 m/s. The induction coil region length in the TekSphero-80kW plasma spheroidization equipment is approximately 0.1 m. Simulation results from the literature indicate that when the coil region length is 0.1 m, the high-temperature zone extending beyond the plasma jet is approximately 0.15 m. Therefore, the high-temperature zone length is set to 0.25 m. The average temperature of the high-temperature zone formed by the plasma torch is difficult to measure experimentally. Consequently, it can only be estimated using numerical simulation results of the spheroidization process from relevant literature. Simulation results indicate that when the coupled power of the induction plasma torch is 15–20 kW, the average temperature of the high-temperature zone is approximately 4000–5000°C. The TekSphero-80kW induction plasma spheroidizing equipment operates at a total power of 60–80kW (the typical power range for spheroidizing production), with the induction plasma torch coupling power being approximately 12–15kW. Consequently, the estimated average temperature of the high-temperature zone is 3000–4000°C. The rate of solid-phase-to-gas-phase transformation is significantly lower than that of liquid-phase-to-gas-phase transformation. Consequently, the primary stage for element vaporization occurs between particle melting and subsequent solidification. Figure 3 illustrates the effect of average plasma torch temperature on Nb element loss rate. Figure 3 includes cases with particle diameters of 30 μm and 50 μm. It is evident that the Nb element loss rate increases significantly with rising temperature. At 3000°C, the Nb element loss rate during the spheroidization of 30 μm particles is approximately 6%, while at 4000°C, the Nb element loss rate approaches 10%.
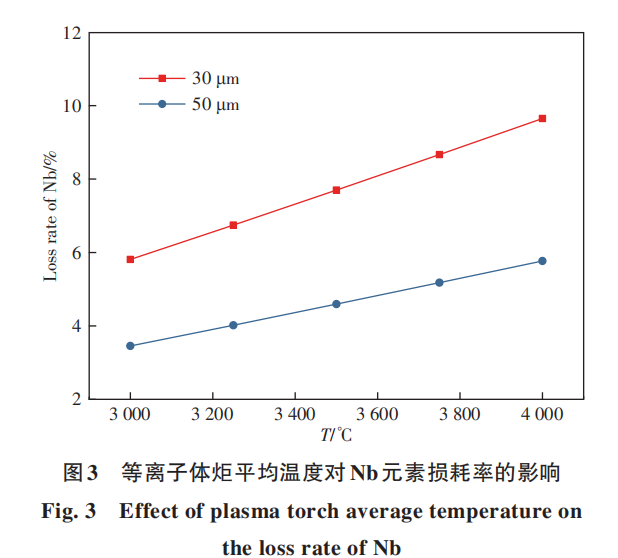
Figure 4 illustrates the effect of particle diameter on Nb element loss rate. It is evident that particle size has a more pronounced impact on Nb element loss rate, with smaller particles exhibiting significantly higher loss rates than larger ones. This occurs because smaller particles possess a larger specific surface area for molten droplets, making elements on the droplet surface more susceptible to vaporization and volatilization. The loss rate increases substantially as particle diameter decreases. At 4000°C, the loss rate for 20μm powder reaches nearly 15%, while that for 60μm powder is only about 5%. Vaporization loss not only reduces the average particle size but also widens the particle size distribution range due to varying vaporization rates across different sizes. Therefore, during raw material preparation, powder size can be slightly increased while controlling the particle size distribution within a narrower range. This ensures the powder meets the size requirements for 3D printing applications after spheroidization.
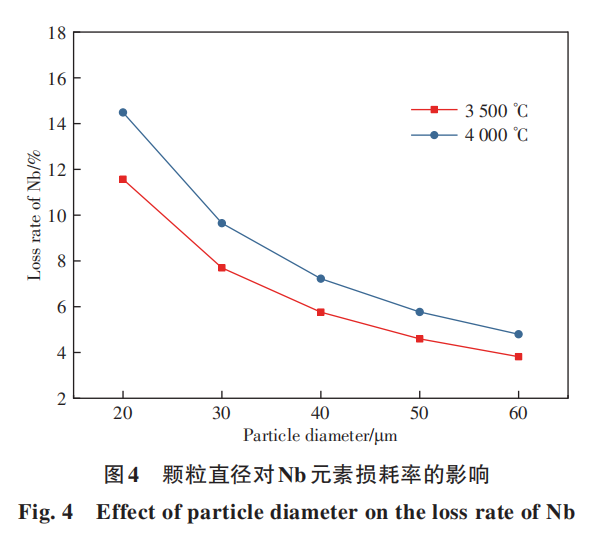
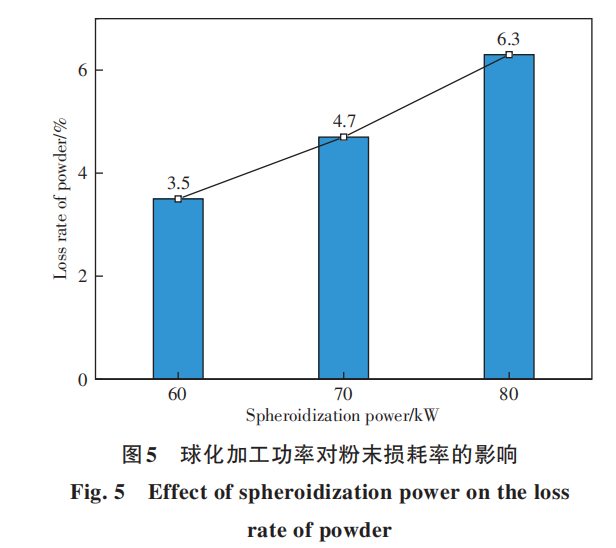
Figure 5 shows the effect of spheroidizing power on powder loss during the spheroidizing process of Nb521 alloy, where the raw material powder has a particle size distribution ranging from 30 to 50 μm. The powder loss rate increases with rising power. At 60 kW, the powder loss rate was only 3.5%, while at 80 kW, it reached 6.3%. Increasing spheroidizing power elevates the temperature within the plasma torch, accelerating the vaporization and volatilization of surface elements in droplets. Theoretical calculations also indicate that as the temperature in the high-temperature zone increases, the element loss rate significantly rises. Table 3 compares theoretical and experimental powder loss rates. The experiments employed spheroidization powers of 60–80 kW, corresponding to average high-temperature zone temperatures of approximately 3000–4000°C. Theoretical calculations used a particle size of 50 μm (derived from Figure 3). Comparison reveals consistent trends in loss rates between the two sets of results. The experimentally obtained loss rate is slightly higher than the theoretical calculation value. This may be attributed to the experimental powder having a particle size range of 30–50 μm, with an average diameter below 50 μm. Previous studies indicate that the loss rate increases as particle size decreases, explaining the higher experimental loss rate. Additionally, comparing the results in Figure 3 shows that the experimental loss rate is lower than the calculated results for particles below 30 μm. During experiments and production, powder lost through vaporization recondenses into solid particles upon cooling. Due to their extremely small diameter (less than 5 μm), these fine particles are carried by the gas flow into the exhaust duct. During this transport, some adhere to the inner walls of the spheroidization chamber and ducts, some separate from the carrier gas at the filtration unit, and others attach to the surface of the spheroidized powder. Due to their extremely small particle size, these powders are unsuitable for additive manufacturing but can be utilized in applications requiring nanoscale powders or recycled for re-melting and re-pulverization. Therefore, during production, while ensuring adequate powder spheroidization rate, processing power should not be excessively high. Appropriate spheroidization power must be selected to maintain both high powder spheroidization rate and high yield.
2.2 Bubble Removal
Bubble removal primarily occurs after particles melt into droplets. Some bubbles originate from within the powder, while others form when elements like O and N separate from alloy elements under high temperatures, precipitating and aggregating. Bubble separation from droplets is a critical step in removing gaseous elements. The methods introduced in Section 1 were used to calculate the bubble removal time during powder spheroidization, focusing on the effects of plasma torch average temperature and particle diameter. The average temperature ranged from 3000 to 4000°C. Since oxygen and nitrogen bubbles have similar densities but differ significantly from niobium density, their removal patterns are largely consistent. Calculations here use oxygen bubbles as an example, with removal time defined as the interval from relative motion between bubble and droplet to complete separation from the droplet surface. Assuming the bubble is located at the center of the droplet, the bubble removal time represents the average time for all bubbles to depart from the droplet.
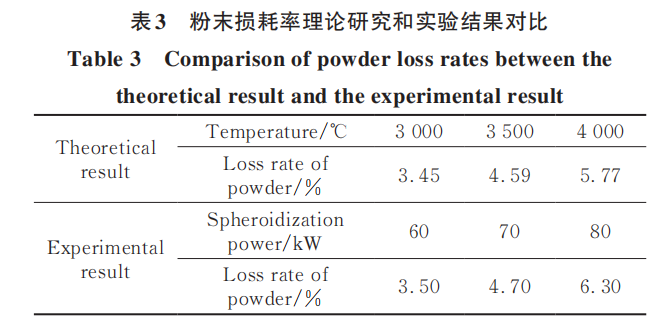
Figure 6 illustrates the effect of average plasma torch temperature on bubble removal time. It shows that bubble separation from droplets occurs extremely rapidly, with the time scale being less than 10⁻⁵ s. As temperature increases, bubble removal time slightly decreases. This is primarily because higher temperatures reduce the viscosity of the molten metal droplets, making bubble removal easier. During spheroidization, particles exist in the liquid state for a time scale of 10⁻¹ s, providing ample time for bubbles to escape from droplets. Therefore, ensuring maximum particle melting during spheroidization is an effective method to enhance bubble removal. Increasing spheroidization temperature and reducing gas flow velocity can both increase the probability of particle melting.
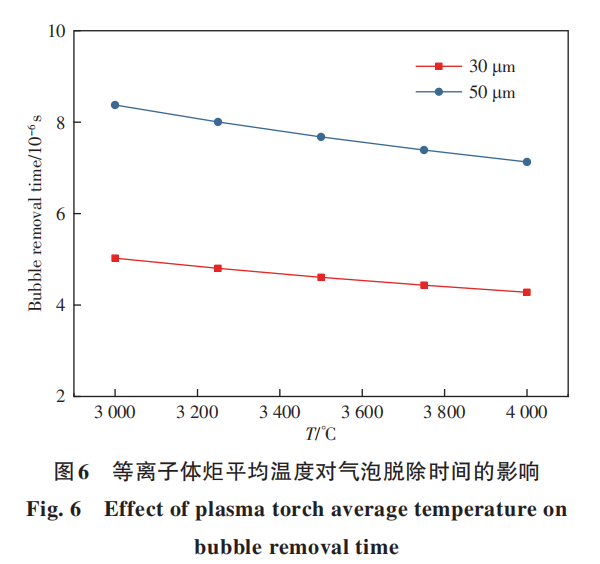
Figure 7 illustrates the effect of particle diameter on bubble removal time at temperatures of 3500 and 4000°C. It is evident that bubble removal time increases significantly with larger particle diameters, exhibiting a near-linear relationship. As particle diameter increases, the average distance between bubbles and droplet surfaces grows, requiring bubbles to travel farther to reach the surface. Consequently, the time required for bubbles to escape from droplets also increases. Although smaller particle sizes facilitate bubble removal, previous research indicates that reducing particle size increases alloy loss rates. Moreover, the bubble removal process occurs extremely rapidly. Larger particles also have ample degassing time after melting. Therefore, there is no need to select finer powders solely to improve bubble removal rates.
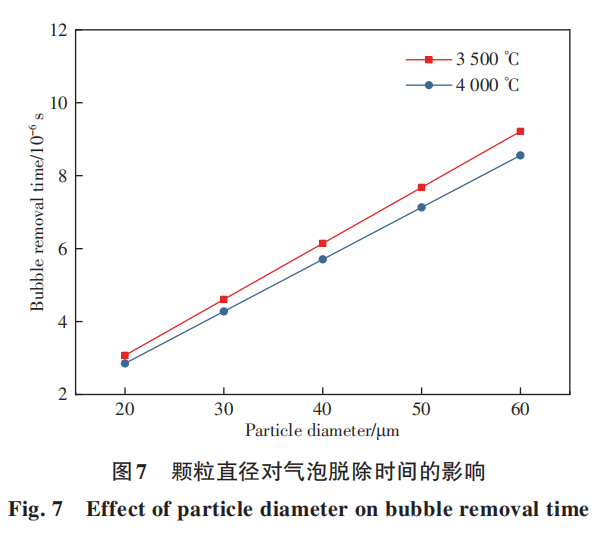
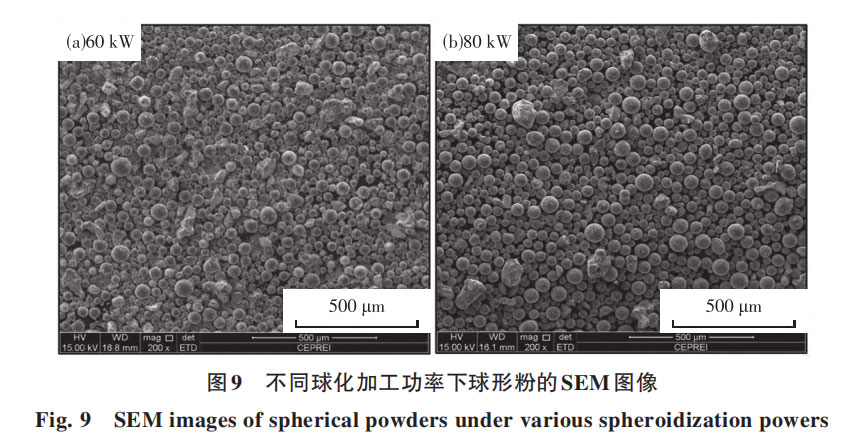
The separation of bubbles and droplets is a critical step in removing gaseous elements such as O and N. This separation process is strongly correlated with deoxidation. Typically, the longer particles remain in droplet form, the shorter the bubble-droplet separation time required, thereby enhancing deoxidation efficiency. Subsequently, experimental studies investigated O concentration changes during the spheroidization process. Figure 8 illustrates the oxygen removal rate during the spheroidization of Nb521 alloy. The spheroidization process demonstrates significant deoxidation effects. At spheroidization powers of 60 kW and 70 kW, oxygen content decreased from an initial 0.038% to 0.008% (though slight variations in oxygen content may exist between the two conditions, detection accuracy is limited to 0.001%). The deoxidation rate reached approximately 79%. At 80 kW, O decreased from the initial 0.038% to 0.007%, with the deoxidation rate slightly increasing to 81.58%. Increasing the spheroidization power can marginally enhance the O removal rate. Based on the previous theoretical calculations, the separation of bubbles and droplets occurs relatively quickly. As long as the particles can be melted, there is sufficient time to complete bubble removal. Therefore, ensuring particle melting is the key to improving the O removal rate. Figure 9 shows SEM images of spherical powders obtained at processing powers of 60 kW and 80 kW. The 80 kW condition exhibits fewer irregular particles and a slightly higher spheroidization rate than the 60 kW condition. This indicates that increased spheroidization power allows more powder particles to melt into small droplets and achieve spheroidization. Consequently, the oxygen removal rate at 80 kW is also slightly higher than at 60 kW. Furthermore, the separation of bubbles from droplets is a critical step for removing gaseous elements like O and N. This separation process is strongly correlated with deoxidation. Typically, the longer particles remain in droplet form, the shorter the bubble-droplet separation time required, which is more conducive to deoxidation. Subsequently, experiments were conducted to investigate O changes during the spheroidization process. Figure 8 shows the O removal rate during the spheroidization of Nb521 alloy. It is evident that the spheroidization process exhibits significant deoxidation effects. At spheroidization powers of 60kW and 70kW, O decreased from an initial 0.038% to 0.008% (though oxygen content may have slight variations between the two conditions, the detection accuracy only reaches 0.001%), achieving a deoxidation rate of approximately 79%. At 80 kW, O decreased from the initial 0.038% to 0.007%, with the deoxidation rate slightly increasing to 81.58%. Increasing the spheroidization power can marginally enhance the O removal rate. As indicated by previous theoretical calculations, the separation of bubbles and droplets occurs relatively quickly. As long as the particles can be melted, there is sufficient time to complete bubble removal. Therefore, ensuring particle melting is crucial for improving the O removal rate. Figure 9 shows SEM images of spherical powders obtained at processing powers of 60 kW and 80 kW. The 80 kW condition exhibits fewer irregular particles and a slightly higher spheroidization rate than the 60 kW condition. This indicates that increased spheroidization power melts more powder particles into small droplets, thereby achieving spheroidization. Consequently, the oxygen removal rate at 80 kW is also slightly higher than at 60 kW. Furthermore, residual oxygen cannot be further removed by increasing power. One reason is that a small fraction of large particles resist melting during spheroidization, limiting oxygen removal. Another reason is that oxygen originally exists as oxides within the alloy. However, the time particles spend in the molten droplet state is brief. Within this timeframe, some oxides cannot fully decompose. This residual oxygen cannot precipitate and agglomerate to form bubbles, preventing separation from the droplets. Consequently, it remains trapped within the powder.
This study also measured nitrogen (N) content in Nb521 powder before and after spheroidization. However, results indicate N levels remained below 0.001% throughout—a negligible concentration that can be considered negligible. Thus, investigating its removal rate holds no practical significance.
3 Conclusions
This study investigated the fundamental mechanisms of Nb loss and bubble removal during plasma spheroidization of Nb alloys. Theoretical analysis examined the effects of temperature and particle size on Nb loss and bubble removal. Subsequently, using Nb521 as raw material, experimental studies explored the influence of spheroidization power on alloy loss rate and O removal rate.
1) Theoretical analysis of alloy element loss rates during spheroidization indicates: Nb loss rate increases significantly with rising temperature, reaching nearly 10% for 30μm Nb particles at 4000°C. Particle size exhibits a more pronounced effect on Nb loss rate, which increases substantially as particle diameter decreases. At 4000°C, the loss rate for 20μm powder particles approaches 15%. Spheroidization experiments confirm this pattern: powder loss rate markedly increases with higher spheroidization power, reaching 6.3% at 80 kW. Therefore, excessively high spheroidization power should not be selected solely to boost spheroidization rate during production. Instead, a balance between spheroidization rate and yield must be sought based on actual conditions.
2) Bubble removal studies indicate: The separation of bubbles from droplets occurs extremely rapidly, taking less than 10⁻⁵ seconds. In contrast, the duration particles remain in a liquid state during spheroidization is on the order of 10⁻¹ seconds, providing ample time for bubbles to escape from droplets. Melting of powder particles is a necessary condition for bubble removal. For coarse-grained powders, increasing melting probability through methods such as raising temperature or reducing carrier gas flow rate are effective measures to promote bubble removal. Experimental results for Nb521 powder spheroidization indicate that increasing spheroidization power slightly enhances the deoxidation rate. Oxygen content decreases significantly during spheroidization, with the deoxidation rate approaching 80%.
Reference: doi: 10.20242/j.issn.2097-5384. 2025. 07. 001 Chinese Library Classification: TU512; TF12 Document Code: A Article ID: 2097-5384(2025)07-1087-09 Alloy Loss and Impurity Removal in Spheroidization of Niobium Alloy Powder for Additive Manufacturing
Ren Binglang1,2, Liu Qi1,2, Bo Xinwei1,2, Wang Xiaoyu1,2, Yao Zhiyuan1,2, Han Xiaoyu1,2, He Haoran1,2
Stardust Technology's spherical niobium alloy powder is produced using radiofrequency plasma spheroidization technology. It features purity exceeding 99.95%, oxygen content below 500 ppm, high sphericity, smooth surface, minimal hollow particles and satellite spheres, excellent flowability, and controllable particle size. The product combines high melting point, good plasticity, and high-temperature specific strength with high bulk and tapped densities. Suitable for processes like selective laser melting (SLM) and injection molding, it can be used for aerospace thermal protection and structural component manufacturing, as well as for producing high-density orthopedic medical parts via 3D printing.For more details, please feel free to contact our professional staff, Manager Cathie Zheng at +86 13318326187.
