
CoCrNi MEAs exhibit excellent mechanical properties, particularly at low temperatures [1–3], and hold promise as a replacement for stainless steel materials in structural components for marine environments [4–5]. However, in marine service environments, high Cl– content can easily cause the passivation film on the surface of medium-entropy alloys to break down, leading to localized corrosion. Therefore, the correlation between the microstructure and corrosion resistance of medium-entropy alloys in service environments is critical for the long-term safe operation of equipment.
In recent years, the corrosion behavior of CoCrNi medium-entropy alloys (CoCrNiMEA) under different service environments has been extensively studied [6–11]. Feng et al. [6] investigated the corrosion behavior of powder metallurgy CoCrNi and CoCrNiN in a 3.5% NaCl (mass fraction) solution, and the results indicated that the corrosion resistance of powder metallurgy medium-entropy alloys was superior to that of commonly used stainless steels (304/316 stainless steel). Wang et al. [9] used spark plasma sintering (SPS) to prepare CoCrNiMEA, which exhibited better corrosion resistance in 1 mol/L H₂SO₄ solution but poorer corrosion resistance in 1.5 mol/L NaOH solution. Moravcik et al. [10] reported a CoCrNiMEA prepared through casting, rolling, and heat treatment, which exhibited superior corrosion resistance to 316L stainless steel in a 0.1 mol/L H₂SO₄ solution. In summary, the corrosion resistance of entropy alloys in CoCrNi varies significantly under different service conditions, primarily due to microstructural inhomogeneity.
With the diversification of equipment structural components, entropy alloys in additive manufacturing are expected to be used for the rapid preparation of complex structural components and in-situ repair of equipment. Weng et al. [12] pointed out that some subgrains and low-angle grain boundaries exist in CoCrNiMEA prepared by laser powder bed fusion (LPBF), and this non-uniform microstructure may lead to different corrosion behaviors and mechanisms. Feng et al. [8] reported that CoCrNiMEA laser cladding coatings exhibit superior corrosion resistance compared to 304 stainless steel in a 0.5 mol/L H₂SO₄ solution. Zhang et al. [7] found that CoCrNi produced via LPBF exhibits inferior corrosion resistance in a 3.5% NaCl (mass fraction) solution compared to cast samples and exhibits noticeable pitting corrosion at room temperature. The differences in corrosion resistance are primarily attributed to manufacturing defects and microstructural uniformity in entropy alloys produced via additive manufacturing. By optimizing process parameters, a relative density exceeding 99.9% can be achieved, thereby eliminating the primary influence of manufacturing defects. Therefore, further research is needed to investigate the correlation between microstructural uniformity and corrosion resistance in entropy alloys produced via additive manufacturing of CoCrNi.
In this study, laser powder bed fusion (LPBF) CoCrNi alloys were annealed at different temperatures to optimize their dislocation cell-like structure and columnar grain structure. The microstructure after different annealing treatments was studied using scanning electron microscopy (SEM), electron backscattering diffraction (EBSD), and transmission electron microscopy (TEM). The corrosion behavior and passivation film composition of LPBFCoCrNi were analyzed using electrochemical testing and X-ray photoelectron spectroscopy (XPS). A reasonable microstructure for optimizing the corrosion resistance of LPBFCoCrNi entropy alloys through heat treatment was proposed, and guidance was provided for post-heat treatment of LPBFCoCrNi entropy alloys.
1 Experimental Materials and Methods
1.1 Materials and Preparation
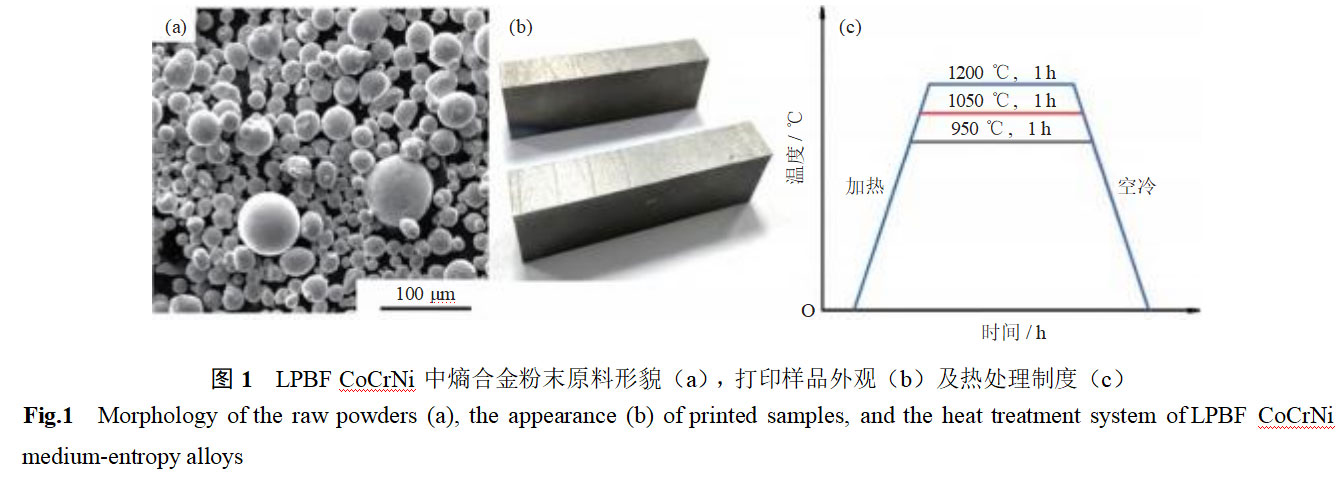
The 3D printing powder material for LPBF CoCrNi medium-entropy alloys is spherical CoCrNi alloy powder, with the main chemical composition (mass fraction) being Co 33.23%, Cr 33.14%, and Ni 33.42%. The powder morphology is shown in Figure 1(a), with an average particle size of approximately 20 μm. The metal 3D printer operates at a laser power of 195 W and a scanning speed of 850 mm/s, with a laser beam width of 0.10 mm and a layer thickness of 0.03 mm. The printing process is conducted under argon gas protection, with the sample dimensions being 20 mm × 10 mm × 10 mm, as shown in Figure 1(b). The relative density of the LPBF CoCrNi medium-entropy alloy was determined to be 99.94% using the drainage method. No obvious manufacturing defects were observed under a 400× optical microscope. The main components of the LPBF CoCrNi medium-entropy alloy are (by mass fraction): Co 33.8700%, Cr 32.1400%, Ni 33.6500%, C 0.0054%, O 0.0200%, N 0.0060%, Si 0.0450%, Cu 0.0120%, and Al 0.0270%. To eliminate internal stresses and homogenize the microstructure, heat treatment was performed at 900, 1050, and 1200°C in an argon atmosphere for 1 hour (air cooling), with the heat treatment regimen shown in Figure 1(c).
1.2 Microstructural Analysis
The microstructure of the samples was observed using an optical microscope and electron backscatter diffraction. The sample size was 10 mm × 10 mm, and after polishing, it was etched using an etching solution (2 g FeCl₃ + 5 mL HCl + 1 mL HNO₃ + 10 mL H₂O). The samples (ϕ3 mm) were ground to 30 μm using SiC sandpaper and then thinned by sputtering with a 5% perchloric acid + 95% acetic acid solution (by volume) at 288 K and 30 V. The microstructure was further observed using a FEI Technai G20 transmission electron microscope and its built-in energy-dispersive spectroscopy (EDS) to further observe the microstructure of the sample and analyze the phase composition and corrosion behavior. The study of microstructure and corrosion behavior was conducted in the XZ plane (perpendicular to the surface of the powder bed). The phase diagram and element distribution of the entropy alloy in CoCrNi were calculated using Thermo-calc phase diagram software under different temperature conditions (800–1300°C).
1.3 Electrochemical Testing
Electrochemical testing was conducted to evaluate the corrosion behavior of LPBF CoCrNi entropic alloys. Electrochemical testing was performed using entropic alloy electrodes with dimensions of 10 mm × 10 mm × 2 mm, embedded in epoxy resin with an exposed area of 1 cm². The sample surface was polished with 2000-grit SiC sandpaper and rinsed with distilled water and anhydrous ethanol. Conventional three-electrode electrochemical testing was conducted using the Donghua Electrochemical Workstation in a 3.5% NaCl solution (mass fraction) at room temperature. The electrode was a platinum plate, and the reference electrode was a saturated calomel electrode (SCE). Before conducting the dynamic potential polarization test, the open-circuit potential (OCP) was tested for 30 minutes to ensure system stability. The potential scanning rate for dynamic polarization was 0.1667 mV·s–1.
The composition of the passivation film formed on the surface of LPBF CoCrNi alloy under different heat treatment conditions was measured using X-ray photoelectron spectroscopy (XPS). The samples were left in air for 30 days to form the passivation film, and a transmission energy of 55 eV was used during testing. All peaks were fitted using XPSPEAK software and corrected using standard peaks (C1s, 284.8 eV).
2 Results and Discussion
Figure 2 shows the entropy-based phase composition and content changes in CoCrNi calculated by Thermo-calc. As shown in the figure, the alloy is primarily a face-centered cubic (FCC) single-phase solid solution. When the temperature is below 879°C, the alloy contains a small amount of carbide precipitation phases, primarily M23C6 and M7C3; when the temperature is above 879°C, M23C6 disappears, and the second phase is primarily M7C3.
Figure 3 shows the microstructure of LPBF CoCrNi entropy alloys after different heat treatments. As can be seen from the figure, the printed samples exhibit distinct melt pool structures, with large grain boundary angles at the melt pool interfaces. As the solution treatment temperature increases from 900°C to 1050°C, the melt pool characteristics gradually weaken, and the proportion of large-angle grain boundaries decreases. When the solution treatment temperature reaches 1200°C, the melt pool structure completely disappears, and the sample exhibits a distinct equiaxed grain structure, with obvious twinning structures visible within the grains. This is primarily attributed to the recovery and recrystallization processes of the microstructure under high-temperature conditions [13–14].
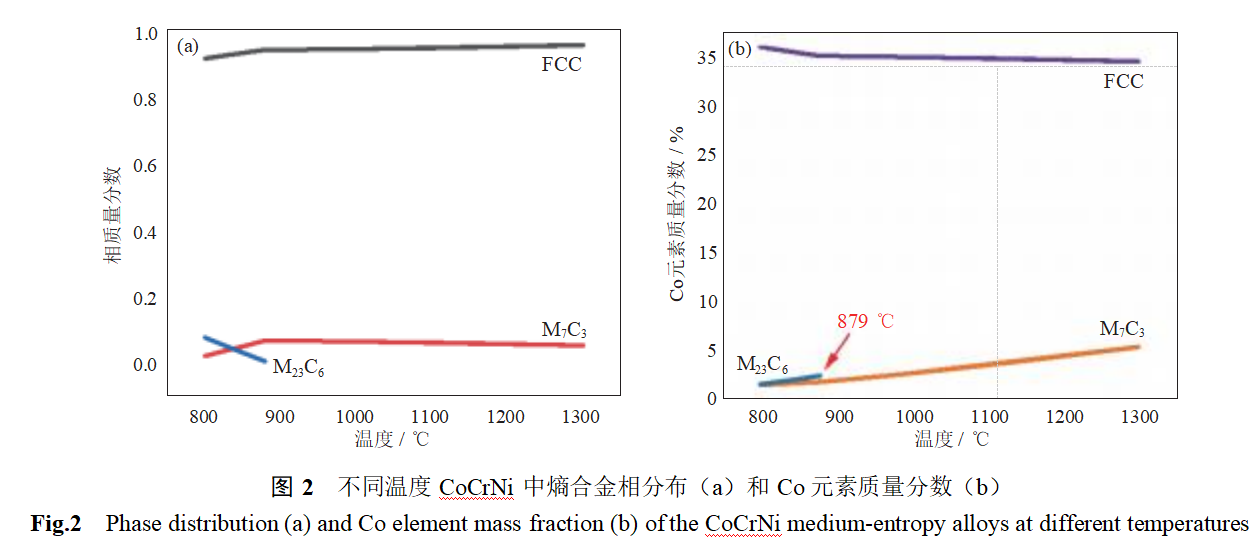
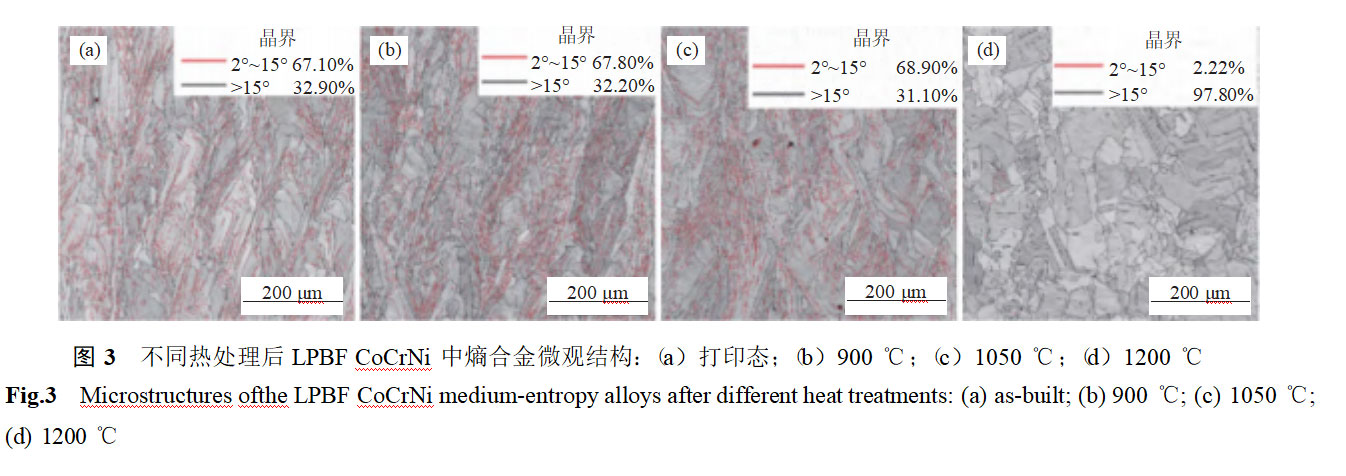 Figure 4 shows the electron backscatter diffraction results of the entropy alloy in LPBF CoCrNi after different heat treatments. In the as-printed samples, a distinct columnar crystal structure is observed, primarily due to the large temperature gradient within the melt pool during the printing process. No obvious grain orientation is detected, and significant stress concentration (dislocation enrichment) is present at the melt pool interface and grain boundaries. As the solid solution temperature increases from 900°C to 1050°C, the columnar crystal structure persists, and no obvious recovery process is observed. The grains exhibit obvious preferred orientation (Figures 5(b)–(c)). When the heat treatment temperature is raised to 1200°C, the columnar crystal structure transforms into an equiaxed crystal structure, with no obvious dislocation enrichment observed at grain boundaries. At this point, the specimen undergoes recovery recrystallization, and no obvious preferred orientation is observed within the grains (Figure 5(d)).
Figure 4 shows the electron backscatter diffraction results of the entropy alloy in LPBF CoCrNi after different heat treatments. In the as-printed samples, a distinct columnar crystal structure is observed, primarily due to the large temperature gradient within the melt pool during the printing process. No obvious grain orientation is detected, and significant stress concentration (dislocation enrichment) is present at the melt pool interface and grain boundaries. As the solid solution temperature increases from 900°C to 1050°C, the columnar crystal structure persists, and no obvious recovery process is observed. The grains exhibit obvious preferred orientation (Figures 5(b)–(c)). When the heat treatment temperature is raised to 1200°C, the columnar crystal structure transforms into an equiaxed crystal structure, with no obvious dislocation enrichment observed at grain boundaries. At this point, the specimen undergoes recovery recrystallization, and no obvious preferred orientation is observed within the grains (Figure 5(d)).
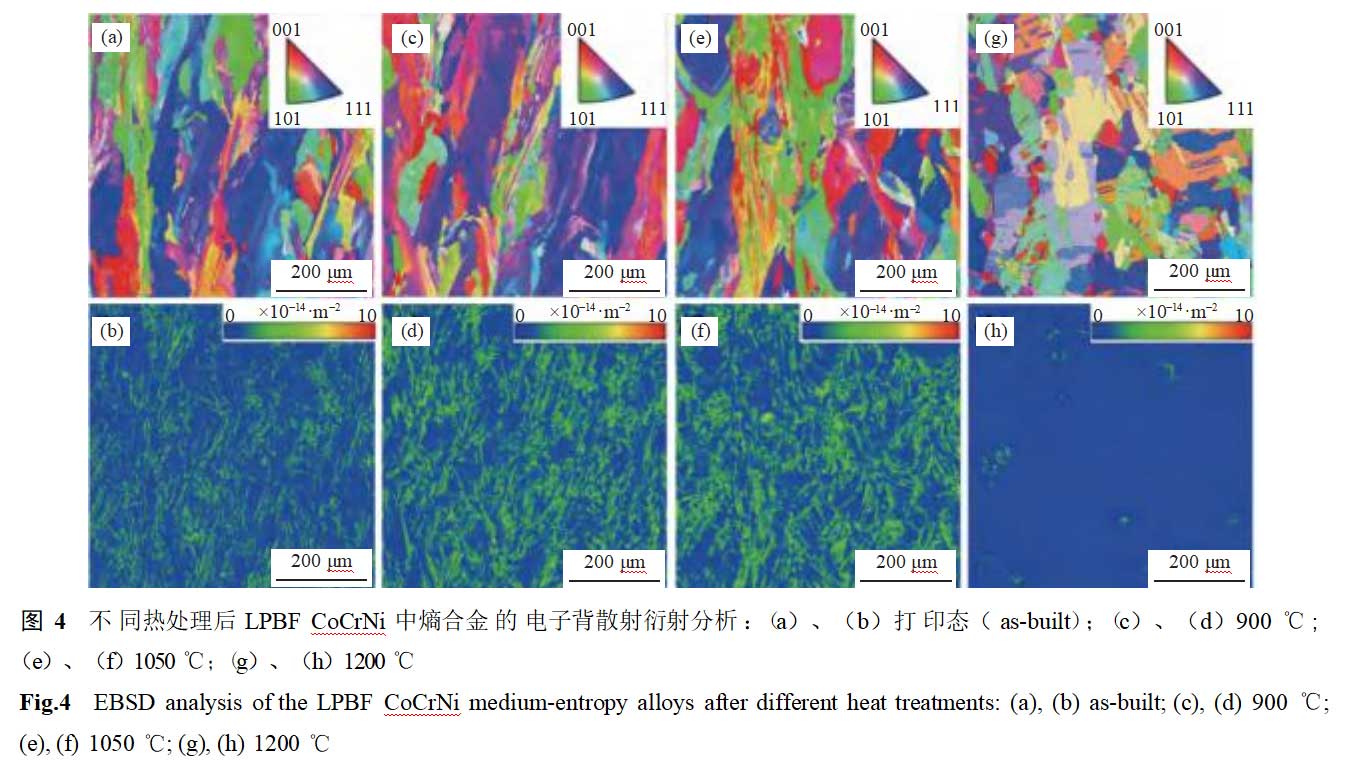
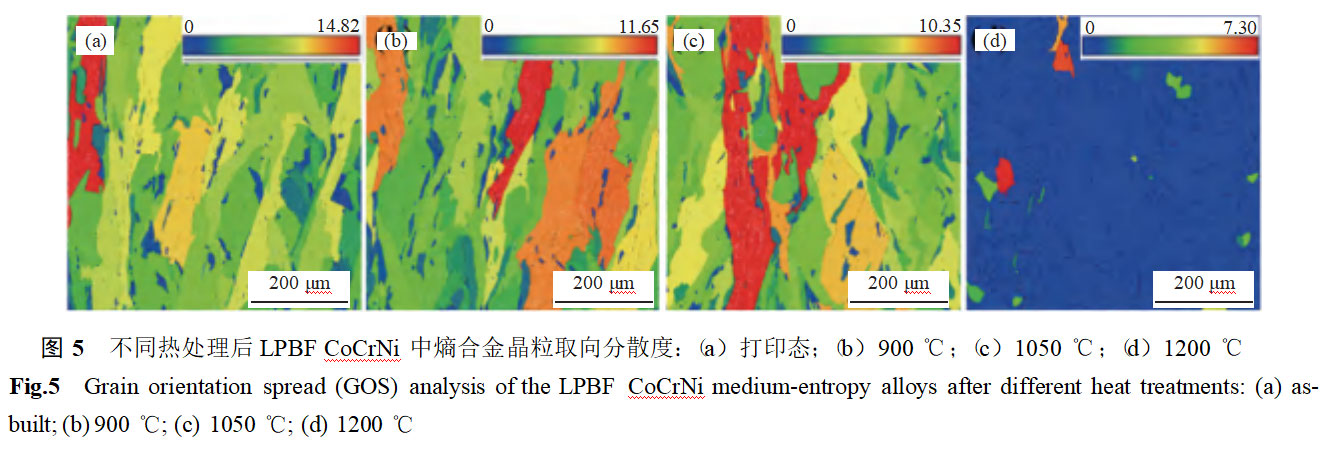
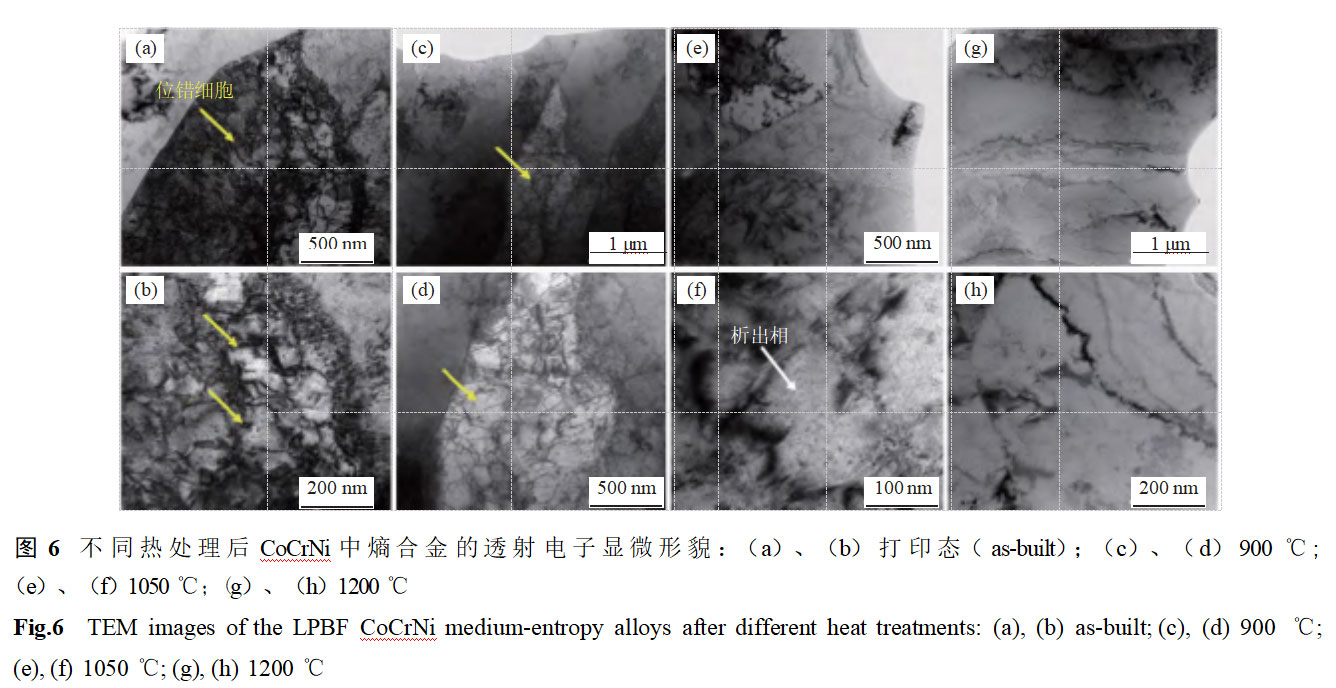
Figure 6 shows the transmission electron microscopy morphology of LPBF CoCrNi alloy after different heat treatments. Printed samples exhibit distinct substructures, with sub-structure boundaries primarily composed of dislocation tangles, and dislocation cell sizes ranging from approximately 100 to 300 nm. As the solid solution temperature increases from 900°C to 1050°C, the dislocation cell structure gradually disappears, primarily due to dislocation movement under high-temperature solid solution conditions, resulting in a gradually more uniform microstructure. Distinct nanoscale precipitates are visible within the sample. Related studies indicate that the primary components of the precipitated phases in CoCrNi alloys are carbides (M23C6, M7C3) [15–16]. When the dissolution temperature reaches 1200°C, the sample has completed the recovery and recrystallization processes, and no obvious dislocation cell structures or precipitated phases are observed in the microstructure, which becomes uniform.
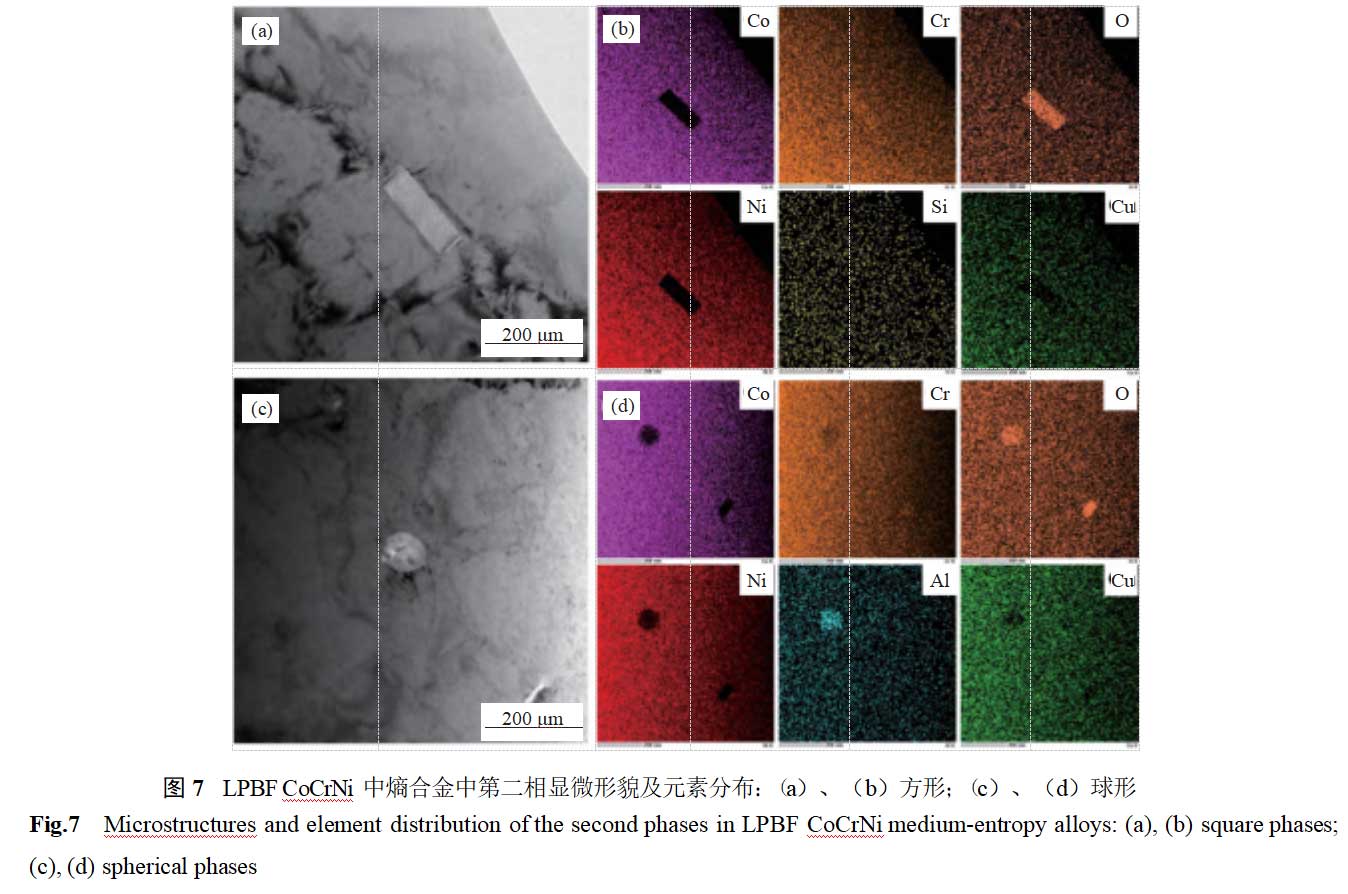
Figure 7 shows the morphology and elemental distribution of the second phase in LPBF CoCrNi entropy alloy. Two distinct morphologies of micro- and nano-sized second phases are present in the microstructure. According to energy dispersive spectroscopy (EDS) analysis, the square-shaped second phase has a size of approximately 200 nm and is primarily composed of chromium oxide; the spherical-shaped second phase has a size of approximately 100 nm and is primarily composed of aluminum oxide. The higher oxide content in the second phase is primarily attributed to oxygen impurities in the spherical powder and oxygen introduced during the additive manufacturing process due to insufficient protective atmosphere [17–18].
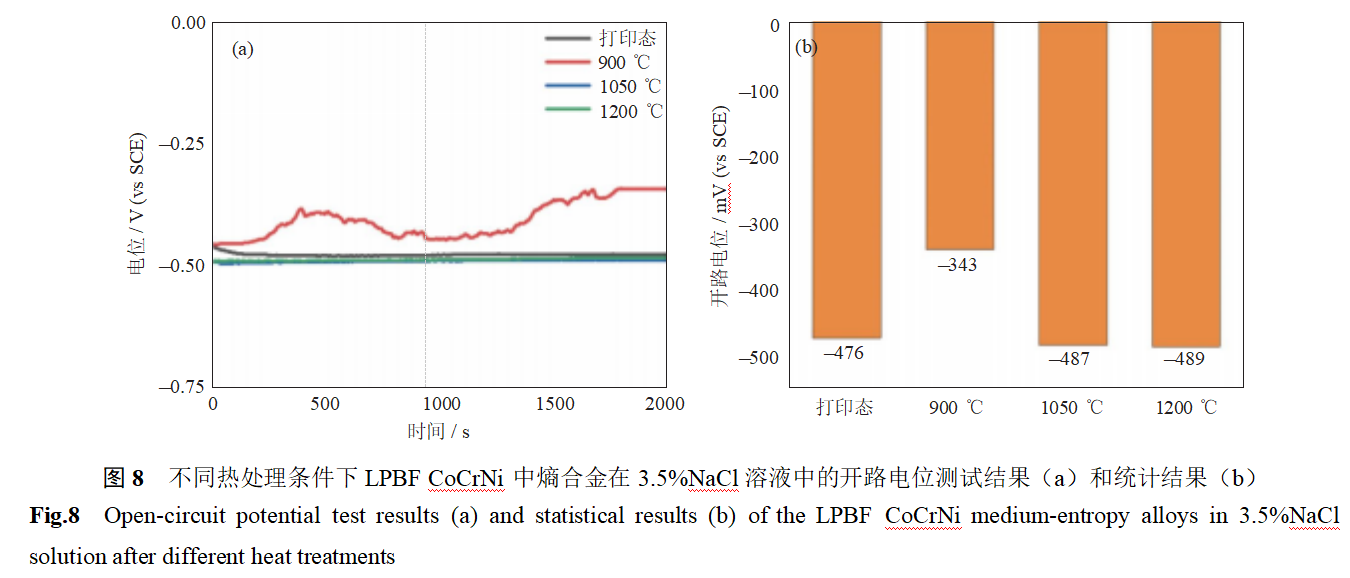
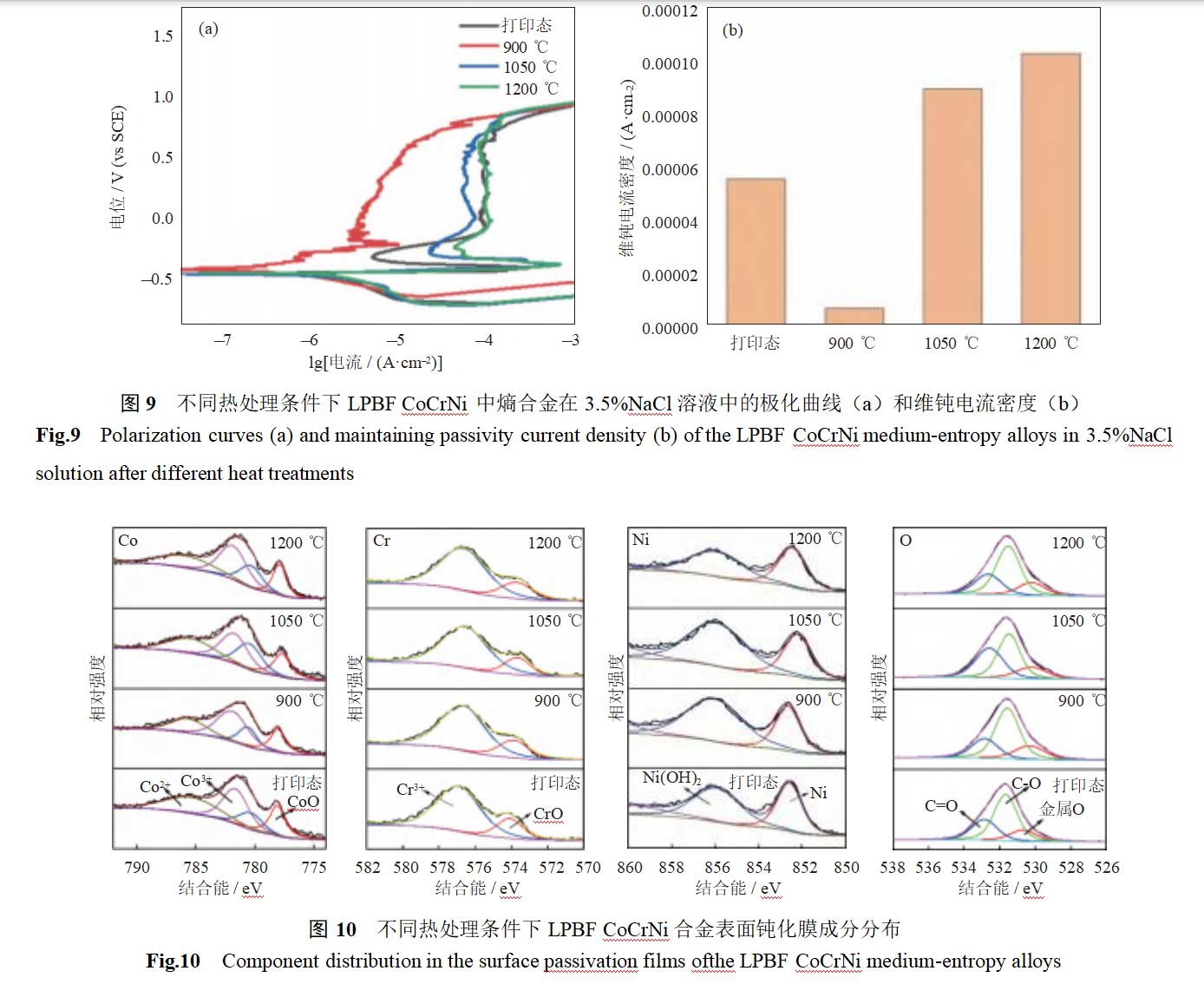
Figure 8 shows the open-circuit potential of LPBF CoCrNi entropy alloys in a 3.5% NaCl solution under different heat treatment conditions. As shown in the figure, the open-circuit potential of CoCrNi entropy alloys in a 3.5% NaCl solution after 3D printing is –476 mV vs. SCE. After heat treatment at 900°C, the open-circuit potential increases to –343 mV vs. SCE. This is primarily attributed to dislocation migration and residual stress release during heat treatment, which enhance the local uniformity of the material. Additionally, high-density cellular dislocation walls accelerate surface reaction kinetics by increasing electronic active diffusion. The higher surface potential of cellular dislocation walls promotes the formation of a passivation film, resulting in improved corrosion resistance [19–20]. As the heat treatment temperature is increased to 1050°C and 1200°C, the open-circuit potential gradually decreases to –487 to –489 mV vs. SCE. This is primarily attributed to the disappearance of the cellular dislocation structure, leading to the loss of element enrichment at the dislocation cell walls, which hinders the rapid formation of the passivation film and results in reduced corrosion resistance.
To further investigate the dynamic stability of the passivation film, polarization curves of LPBF CoCrNi alloy were measured in a 3.5% NaCl solution under different heat treatment conditions, as shown in Figure 9. As can be seen from the figure, all polarization curves exhibit distinct passivation intervals, indicating that the alloy spontaneously forms a passivation film on its surface in simulated seawater solutions [21]. When the heat treatment temperature was 900°C, the polarization curves of the specimens did not exhibit an activation-passivation interval. This indicates that the passivation film formed rapidly in this specimen, with a passivation current density of 10–5.206 A·cm–2, significantly lower than that of other specimens. Additionally, the corrosion potential was higher, the corrosion current density was lower, and the corrosion resistance was better. It can be concluded that after 900°C heat treatment of the additive manufacturing CoCrNi alloy, some dislocations migrate and residual stress disappears, improving corrosion resistance. As the heat treatment temperature increases to 1050°C and 1200°C, the passivation current density is approximately 10–4 A·cm–2, increasing by one order of magnitude, and the polarization curve exhibits a distinct activation-passivation interval. It can be concluded that as the potential increases during polarization curve testing, the specimen enters the activation zone, followed by the formation of the activation-passivation zone, where the passivation film gradually forms [22–23]. It can be concluded that after high-temperature heat treatment, the specimen’s cellular dislocations disappear, columnar grains gradually transform into equiaxed grains, and the specimen’s corrosion resistance decreases.
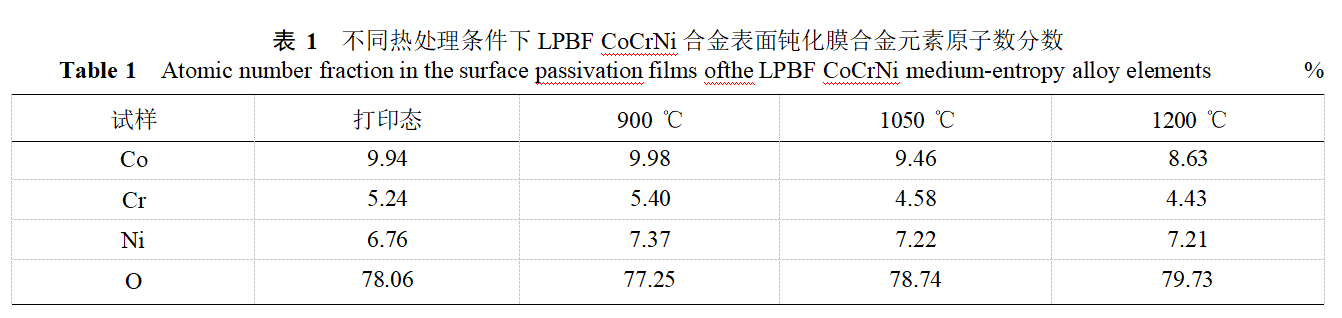
To further investigate the relationship between the alloy's corrosion resistance and the composition of the passivation film, the composition of the passivation film on the surface of LPBF CoCrNi alloy under different heat treatment conditions was analyzed, as shown in Figure 10. As shown in the figure, the passivation film on the surface of the LPBFCoCrNi alloy is primarily composed of metal oxides and metal hydroxides, including: Co²⁺, Co₃O₄²⁺, Cr₂O₃, Cr⁶⁺, Cr(OH)₃, NiO, and Ni(OH)₂. According to the quantitative analysis of alloy elements in Table 1, the passivation film contains the highest proportions of cobalt oxide and hydroxide. Comparing the electrochemical results with the quantitative results, it is found that when the heat treatment temperature is 950°C, the atomic percentage of alloy elements in the passivation film is the highest, approximately 22.75%; as the heat treatment temperature increases to 1050–1200°C, the content of alloy elements in the passivation film is approximately 21.00%.
In summary, when the alloy content in the passivation film on the surface of the LPBF CoCrNi alloy is high, the passivation film has higher density and better corrosion resistance. Additionally, Cr and Ni are the primary elements influencing the corrosion resistance of the passivation film. After heat treatment at 900°C, the Cr content (atomic percentage) in the passivation film is highest, approximately 5.40%, and the Ni content is highest, approximately 7.37%, resulting in good corrosion resistance. As the heat treatment temperature increases, the Cr and Ni content in the passivation film gradually decreases, leading to reduced corrosion resistance. Therefore, the corrosion resistance of LPBF CoCrNi alloy under different heat treatment conditions is related to the alloy composition in the passivation film.
3 Conclusions
(1) After printing, the CoCrNi alloy exhibits a distinct columnar grain structure and dislocation cell-like structure. As the heat treatment temperature increases from 900°C to 1200°C, the dislocation cell-like structure gradually disappears, and the columnar grains gradually transform into equiaxed grains, undergoing recovery recrystallization, with grain size decreasing and the second phase precipitation gradually coarsening.
(2) After heat treatment at 900°C, the corrosion potential is highest, the corrosion current density and passivation current density are lowest, and corrosion resistance is best. This is primarily due to the disappearance of residual stress from the printing process and the promotion of passivation film formation by the dislocation cell-like interface. As the heat treatment temperature increases to 1050–1200°C, the dislocation cell structure disappears, columnar crystals transform into equiaxed crystals, the precipitated phase gradually coarsens, and corrosion resistance decreases.
Reference: Volume 43, Issue 4, Powder Metallurgy Technology Enhancing the corrosion resistance of laser powder bed fusion CoCrNi entropic alloys through microstructural control
CoCrNi alloy powder (typically with an equimolar or near-equimolar ratio) is a medium/high-entropy alloy powder with a face-centered cubic (FCC) single-phase solid solution structure. It exhibits excellent mechanical properties, good corrosion resistance, and wear resistance. It can spontaneously form a passivation film in a 3.5 wt.% NaCl solution, with a low corrosion current density. The powder has high sphericity, low oxygen content, and good flowability, making it suitable for processes such as laser cladding, 3D printing, and thermal spraying. It is widely applied in fields such as aerospace, marine engineering corrosion-resistant and wear-resistant coatings, medical devices (e.g., artificial joints), and surface strengthening of automotive components.
