
High-entropy alloys are high-performance metallic materials developed in recent years by introducing multiple primary elements. They consist of five or more main elements, each present at 5 wt% to 35 wt% [1]. The multi-component mixing in high-entropy alloys achieves high mixed entropy, thereby suppressing the formation of complex secondary phases and resulting in a simple structure. Due to their excellent mechanical properties and chemical stability, high-entropy alloys have become a hot research topic among scholars [2-3]. High-entropy alloys can be broadly categorized into two types: one type primarily consists of elements with relatively low melting points such as Ni, Co, Fe, Cr, Cu, Mn, and Ti, forming FCC solid solution structures; the other type primarily consists of refractory metals such as Mo, Zr, Nb, Hf, Ta, and W, combined with some elements from the fourth period such as V, Ti, and Cr, forming BCC solid solution structures, known as refractory high-entropy alloys [4]. Researchers have also enhanced alloy properties by adding rare elements or adjusting primary element ratios. Past studies predominantly focused on low-melting-point elements, with limited research on refractory high-entropy alloys. The random, disordered distribution of high-melting-point elements within the solid solution confers excellent properties on refractory high-entropy alloys, including high hardness, high strength, high wear resistance, high-temperature oxidation resistance, and thermal stability [5]. Senkov et al. [6] investigated two equiatomic ratio refractory high-entropy alloys, NbMoTaW and NbMoTaWV, discovering both exhibit single-phase body-centered cubic (BCC) structures. At room temperature, they demonstrate high yield strengths of 1058 and 1246 MPa, respectively, outperforming the conventional Inconel 718 nickel-based alloy. Zhao Yizhen et al. [7] fabricated NbMoTa-based refractory high-entropy alloys via selective laser melting. These alloys exhibited a single body-centered cubic structure and demonstrated outstanding room-temperature yield strength and compressive strength.
The preparation methods for high-entropy alloys vary depending on their intended applications. Vacuum arc melting is typically chosen for producing three-dimensional bulk alloys; however, the high cost of manufacturing bulk high-entropy alloys makes large-scale production challenging [8]. Two-dimensional high-entropy alloy coatings are generally produced via magnetron sputtering and laser cladding. Coatings fabricated by magnetron sputtering tend to be thin, exhibit low adhesion to substrates, and are prone to peeling [9]. Additionally, powder high-entropy alloys are primarily prepared using ball milling [10]. The constituent elements of refractory high-entropy alloys possess high melting points with significant inter-element temperature differences. This leads to macroscopic compositional segregation, coarse microstructures, and defects during bulk alloy fabrication, particularly pronounced in large-scale alloy production [11]. Laser cladding technology, however, utilizes metal powders as raw materials. The high energy output from the laser rapidly melts the powder, forming a molten pool on the substrate surface. This pool then solidifies rapidly to create a dense, uniform cladding layer [12-13]. The high power of laser cladding rapidly melts high-melting-point elements like Ta, Nb, and Mo, achieving fast solidification rates during processing. Alloys produced via laser cladding exhibit fine grain sizes and excellent properties such as hardness and tensile strength [14]. Composition can be flexibly adjusted to meet specific requirements, forming high-performance coatings on metal surfaces while conserving materials. Therefore, this study employed laser cladding technology to deposit a refractory high-entropy alloy coating composed of MoNbTaTiV onto a Q235 steel substrate. The microstructure, hardness, wear resistance, and corrosion resistance of the coating were investigated.
1 Experimental Materials and Methods
Metal powders of Mo, Nb, Ta, Ti, and V with an average particle size of 20–50 μm and a purity of 99.9 wt% were selected. These were mixed in an equimolar ratio and blended in a ball mill under an Ar atmosphere for 120 hours. Figure 1 shows the XRD pattern of the mixed MoNbTaTiV metal powder after grinding. It can be observed that after 120 hours of ball milling, the powder phases primarily consist of individual metallic elements without alloying. The substrate consisted of a Q235 steel block measuring 50 mm × 50 mm × 7 mm. The substrate was polished with 400- and 800-grit sandpaper to remove the oxide layer, ensuring a flat surface with adequate roughness to minimize impurity effects and reduce potential laser reflection. The surface was then ultrasonically cleaned with acetone and alcohol 2–3 times to eliminate residual impurities.
Prior to the experiment, the powder should be placed in a drying oven at 120°C for 2 hours to remove moisture adsorbed on the alloy powder surface, ensuring the powder's flowability during feeding. A single cladding layer was deposited on the surface of Q235 steel using a YLS-10000 laser system. Prior to the experiment, high-purity argon gas was introduced for 30 seconds to prevent oxidation of the cladding layer. Laser cladding parameters: laser output power 3000 W, scanning speed 5 mm/s, powder feed rate 15 g/min, spot diameter 3 mm.
The clad layer was machined into 10 mm × 10 mm × 7 mm specimens via wire cutting. Specimens were sequentially ground and polished using 400- to 2000-grit abrasive paper on a P-2T metallographic grinding and polishing machine, followed by 15 seconds of aqua regia etching (concentrated HNO₃: concentrated HCl = 1:3 by mass). The phase composition and morphology of the coating were analyzed using a Bruker AXSD8 Advance X-ray diffractometer (XRD) and a TES-CANMIRALMS scanning electron microscope (SEM). Hardness testing was performed using an HV-1000 microhardness tester at a load of 1.96 N and a loading time of 10 s, with measurements taken at 0.4 mm intervals from the coating surface to the substrate. Wear resistance was evaluated using an MFT-5000 friction and wear tester with 6 mm diameter Al₂O₃ ceramic balls as the counterface material, a load of 30 N, and a test duration of 20 min. The corrosion resistance of the refractory high-entropy alloy coating was investigated using a DH7001 electrochemical workstation. Experiments were conducted in a 3.5 wt% NaCl solution within a three-electrode system: the working electrode was the refractory high-entropy alloy coating, the reference electrode was a saturated calomel electrode, and the counter electrode was a platinum plate. For electrochemical impedance spectroscopy experiments, the starting frequency was set at 105 Hz, the ending frequency at 10⁻² Hz, and the excitation signal was a sinusoidal alternating current with an amplitude of 0.01 V.
2 Experimental Results and Analysis
2.1 Phase Analysis
In physical metallurgy, the Hume-Rothery criterion is primarily used to investigate the effects and patterns of displacement solid solutions in binary alloys. Zhang et al. [15] extended the Hume-Rothery criterion to high-entropy alloys, proposing several key parameters to determine whether a single solid solution phase forms. The atomic size difference δ, mixing enthalpy ΔHmix, and Ω are calculated using Equations (1), (2), and (3), respectively. Empirical data indicate that a single solid solution phase is more likely to form when the mixing enthalpy ΔHmix falls within -15 to 5 kJ/mol, Ω > 1.1, and δ < 6.6%. Guo et al. [16] proposed that the crystal structure of solid solutions can be determined by the valence electron concentration (VEC). Equation (4) calculates the system's VEC, indicating that BCC solid solution phases are relatively stable when VEC ≤ 6.87.
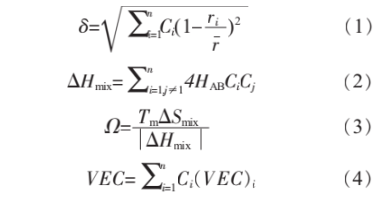
Where: HAB is the interatomic mixing enthalpy; Ci and Cj are the molar fractions of elements i and j, respectively; ri is the atomic radius of element i; r is the average atomic radius; Tm is the average melting point. Interatomic mixing enthalpies are listed in Table 1 [17].
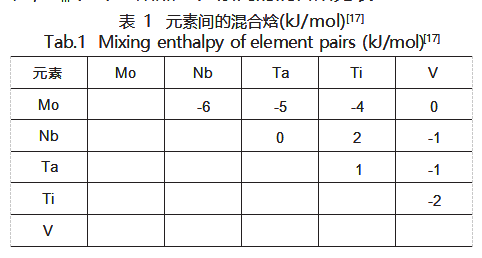
The results for ΔHmix, δ, Ω, and VEC of the refractory high-entropy alloy coating MoNbTaTiV are shown in Table 2. It can be seen that the values of these parameters all satisfy the conditions for the formation of a single BCC solid solution. To further determine whether a simple solid solution phase has formed, it is necessary to calculate the formation free energy of the solid solution in the alloy system. To simplify calculations, based on the assumption by Takeuchi and Inoue, the free energy change (ΔG) in an alloy with fixed composition is linearly proportional to its liquid-phase mixing free energy change (ΔGmix) [18]. Thus, the formation free energy of the solid solution in the alloy system can be expressed by Equation (5):
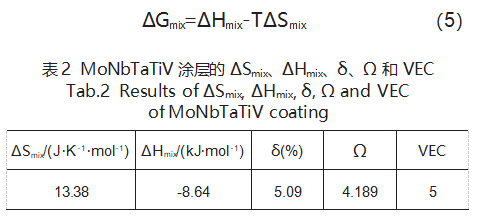
Where: ΔHmix and ΔSmix are the mixing enthalpy and mixing entropy of the solid solution; T is the thermodynamic temperature.
Using Thermal-Calc software to calculate the solidification temperature of the refractory high-entropy alloy MoNbTaTiV, the alloy solidifies completely at 1680 K. According to Equation (5), the formation free energy of a simple solid solution at 1680 K is calculated to be -25.04 kJ/mol. Considering that laser cladding partially dilutes the substrate, the formation enthalpy of intermetallic compounds must account for Fe. Among Mo, Nb, Ta, Ti, V, and Fe, the formation enthalpy of Fe₂Ti (-22.67 kJ/mol) is closest to the formation free energy of the refractory high-entropy alloy MoNbTaTiV (-25.04 kJ/mol).
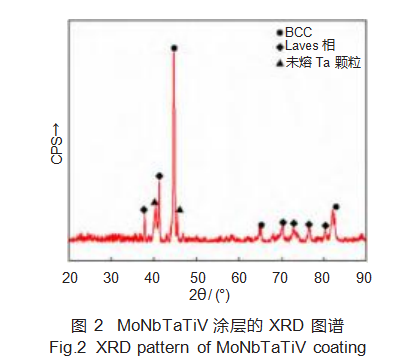
Figure 2 shows the XRD results for the MoNbTaTiV coating. The phase structure of high-entropy alloys is typically determined by calculating the sine values of each diffraction peak to distinguish between BCC and FCC structures [19]. The diffraction peaks at 2θ = 44.727°, 65.188°, 76.536°, and 89.864° in Figure 2 satisfy the proportional relationship sin2θ1:sin2θ2:sin2θ3:sin2θ4 = 2:4:6:8. Based on the crystal diffraction extinction principle, these peaks correspond to the BCC phase. Unmelted Ta particles are also present in the coating due to Ta's extremely high melting point, preventing complete melting by the laser energy during cladding. Additionally, a small amount of Laves phase (Fe₂Ti) formed in the coating, consistent with the aforementioned calculation results.
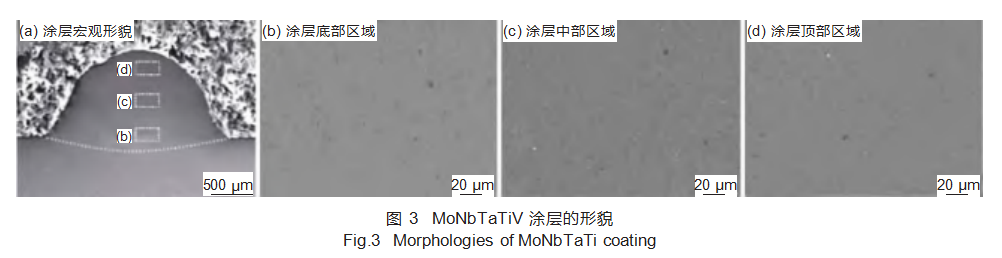
2.2 Microstructural Analysis
Figure 3 shows the microstructural morphology of a cross-section of the MoNbTaTiV refractory high-entropy alloy coating. The coating structure primarily consists of columnar dendrites and equiaxed grains, with columnar dendrites dominating the bottom and middle regions, while equiaxed grains predominate in the top region. The evolution of dendritic morphology within the coating structure is primarily governed by the magnitude of liquid-solid interface undercooling during solidification. In the MoNbTaTiV refractory high-entropy alloy, increasing composition undercooling induces a transition from columnar dendrites to equiaxed grains. It can be observed that grain size progressively refines from the bottom to the top region of the coating. This occurs because laser cladding induces varying temperature gradients and solidification rates across different areas of the clad layer. Closer to the top, the temperature gradient diminishes while the solidification rate increases, resulting in the smallest grain size in the top region of the coating.
Energy dispersive spectroscopy (EDS) analysis of the gray-black particles in the MoNbTaTiV refractory high-entropy alloy coating revealed an Fe:Ti atomic ratio close to 2:1. This occurs because higher laser power melts part of the substrate material, allowing some Fe atoms to enter the melt pool. Combined with XRD and thermodynamic calculations, the gray-black particles are identified as the intermetallic compound Fe₂Ti, consistent with computational results.
2.3 Hardness and Wear Resistance of the Coating
Figure 4 shows the hardness distribution curve of the laser-clad MoNbTaTiV refractory high-entropy alloy coating. The coating exhibits an average hardness of 756.7 HV0.2, which is four times that of the substrate (189 HV0.2) and significantly higher than the base material.
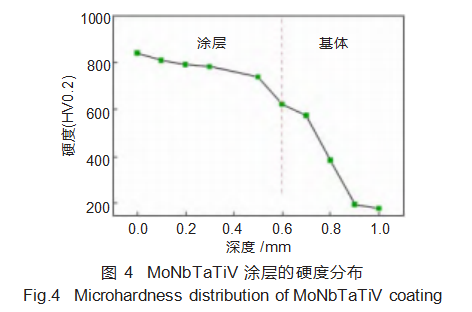
This is partly attributed to the coating's predominant BCC phase structure, which enhances lattice distortion effects within the alloy, markedly increasing hardness. Additionally, a small amount of the intermetallic compound Fe₂Ti formed in the coating, though its quantity was insufficient to significantly boost hardness. Another factor is the rapid solidification rate during laser cladding. Atoms lack sufficient time to diffuse during solidification, limiting nucleation growth and resulting in fine grains that enhance coating hardness. It can be observed that the coating hardness gradually decreases from the top downward. This is because under the high-energy laser beam, the temperature gradient at the top of the coating is smaller, resulting in faster cooling and finer grains. As the temperature gradient increases toward the bottom and middle of the cladding layer, the grains coarsen, leading to reduced hardness.
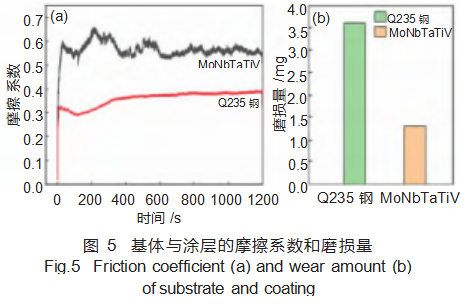
Figure 5 compares the friction coefficients and wear rates between the MoNbTaTiV refractory high-entropy alloy coating and the Q235 steel substrate. During friction and wear testing, both the coating and substrate exhibited friction coefficients that initially increased, then decreased, before stabilizing. The coating's average coefficient of friction was 0.56, slightly higher than the substrate's average (0.37) (Figure 5(a)). However, the coating's wear loss was 1.3 mg, lower than the substrate's 3.6 mg (Figure 5(b)). Generally, higher hardness correlates with lower wear loss and superior wear resistance. This indicates that the MoNbTaTiV refractory high-entropy alloy coating exhibits better wear resistance than the Q235 steel substrate.
2.4 Corrosion Resistance of the Coating
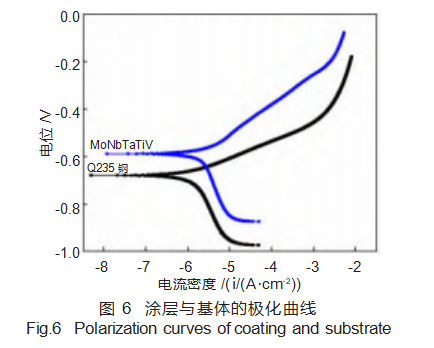
Figure 6 shows the dynamic potential polarization curves of the MoNbTaTiV refractory high-entropy alloy coating and substrate in a 3.5 wt% NaCl solution. During electrochemical testing, Cl⁻ readily reacts with metallic elements in the coating, forming a corrosion product film that penetrates the coating surface and affects material corrosion resistance [20]. It can be observed that the MoNbTaTiV refractory high-entropy alloy coating exhibits a narrow passivation region, indicating the formation of a passivation film. Corrosion continues once this passivation film is breached by Cl⁻. This occurs because Ta, Mo, and Ti in the MoNbTaTiV refractory high-entropy alloy coating exhibit strong corrosion resistance. During electrochemical testing, a passivation layer forms, thereby inhibiting further corrosion.
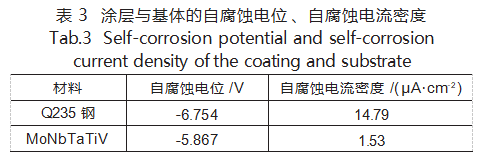
Table 3 presents the self-corrosion potential (Ecorr) and self-corrosion current density (Jcorr) of the coating and substrate, calculated after Tafel fitting of the dynamic polarization curves. The results indicate that both the self-corrosion potential and self-corrosion current density of the MoNbTaTiV refractory high-entropy alloy coating are lower, demonstrating superior corrosion resistance and protective capability for the substrate.
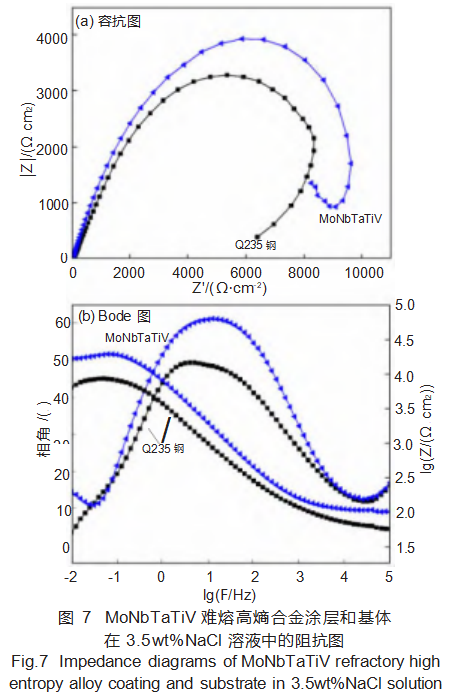
Further electrochemical impedance spectroscopy (EIS) studies were conducted on the coating and substrate. Figure 7 shows the EIS results for the MoNbTaTiV refractory high-entropy alloy coating and substrate in a 3.5 wt% NaCl solution.
Figure 7(a) reveals that the capacitive arc radius of the MoNbTaTiV refractory high-entropy alloy coating is larger, indicating higher charge transfer resistance during corrosion and superior corrosion resistance compared to the substrate. Figure 7(b) presents the Bode plots for the coating and substrate. The relationship between frequency, impedance modulus, and phase angle reveals that the MoNbTaTiV refractory high-entropy alloy coating exhibits a higher impedance modulus, indicating superior corrosion resistance compared to the substrate. The phase angle of the coating approaches 90°, signifying a denser and more stable passivation film, which enhances its corrosion resistance.
3 Conclusions
(1) The coating phase consists primarily of a BCC solid solution and a small amount of the intermetallic compound Fe₂Ti. The coating microstructure is mainly composed of columnar dendrites and equiaxed grains.
(2) The average coating hardness reached 756.7 HV0.2, four times that of the substrate. The coating friction coefficient was slightly higher than the substrate, but wear volume was significantly reduced, demonstrating superior wear resistance compared to the Q235 steel substrate.
(3) In a 3.5 wt% NaCl solution, the coating exhibits a larger arc radius, a more positive self-corrosion potential, and a self-corrosion current density one order of magnitude lower than the substrate. The coating's corrosion resistance is markedly superior to the substrate, providing protective effects.
References: Citation Format: Ao Weibin, He Muyang. Study on Microstructure and Properties of Laser Cladding MoNbTaTiV Refractory High-Entropy Alloy Coatings [J]. Heat Treatment Technology, 2025, 54(22): 87-91+97.
DOI: 10.14158/j.cnki.1001-3814. 20223483 http://www.rjggy.net
Stardust Technology's spherical refractory high-entropy alloy powder is produced via radiofrequency plasma spheroidization. It features high purity with low oxygen content, high sphericity, smooth surfaces free of satellite particles, and uniform particle size distribution. It exhibits high bulk and tapped densities with excellent flow properties. The product is compatible with various processes including laser/electron beam additive manufacturing, hot isostatic pressing, and laser cladding. It finds applications in aerospace, cemented carbides, welding materials, and other fields. With stable and controllable quality, it supports customization across multiple particle size specifications ranging from 1-150μm. Additionally, Xingchen Technology provides tailored composition solutions based on customer requirements and offers 3D printing services. For further details, please contact our professional staff: Manager Cathie Zheng at +86 13318326187.
