
Tantalum is a "biophilic" transition metal that has been widely used in clinical practice as a medical implant material for nearly 80 years [1-2]. In recent years, with the rapid development of materials science, medical porous materials have gradually replaced solid metals and have been used in a variety of surgical implants, and their applications have become more and more extensive and their usage has increased. Porous tantalum has stood out among many medical materials and has become one of the most ideal implant materials due to its excellent properties, such as good bone conduction, bone induction and osteogenesis [3-5]. 3D printing is a rapid prototyping technology with the advantages of personalization and high precision. It is widely used in the biomedical field. Among them, 3D printed personalized implants are a new trend in the development of clinical medicine [6-7]. Based on this, this study conducted a comprehensive biosafety evaluation of 3D printed porous tantalum in accordance with the GB/T 16886 "Biological Evaluation of Medical Devices" standard, aiming to provide a scientific basis for the safe application of this material in the field of surgical implants.
1 Materials and methods
1.1 Experimental materials, instruments and animals
1.1.1 Experimental materials
3D printed porous tantalum, tantalum discs (diameter 10 mm, thickness 1 mm), and titanium discs (diameter 10 mm, thickness 1 mm) were donated by the Department of Orthopaedics, Southwest Hospital, Army Medical University; mouse fibroblasts (L929) and Chinese hamster lung cells (CHL cells) were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai); TA97, TA98, TA100, TA102, TA1535 strains and rat liver microsomal enzyme S9 were purchased from CHI SCIENTIFIC, mitomycin C was purchased from MYM BIOLOGICH TECHNOLOGY, and cyclophosphamide was purchased from SIGMA-ALDRICH, colchicine were purchased from Chongqing Chuandong Chemical (Group) Co., Ltd.; high-density polyethylene film (Lot. No. C-161) and polyurethane film of zinc diethyldithiocarbamate (Lot. No. A-182K) were purchased from Hatano Research Institute, FDSC (Tokyo, Japan); soybean oil (for injection) was purchased from Guangzhou Baiyunshan Hanfang Modern Pharmaceutical Co., Ltd.; 0.9% sodium chloride injection was purchased from Sichuan Kelun Pharmaceutical Co., Ltd.
1.1.2 Experimental instruments
HVA-85 autoclave (Hirayama, Japan), Synergy HTX microplate reader (Biotek, USA), CB150 carbon dioxide incubator (BINDER, Germany), Sorvall ST 8R high-speed refrigerated centrifuge (Thermo Fisher, USA), MJ-250F-Ⅲ microbial incubator (Shanghai Longyue Instrument Equipment Co., Ltd.), IX71 inverted biological microscope (Olympus, Japan), TBA-40FR fully automatic biochemical analyzer (Toshiba, Japan), BC-5300Vet blood analyzer (Shenzhen Mindray Bio-Medical Electronics Co., Ltd.), UV2600 spectrophotometer (Shimadzu, Japan), UX620H electronic balance (himadzu, Japan), ACS-6Kg electronic price scale (Yongkang Yongzhou Weighing Instrument Co., Ltd.).
1.1.3 Experimental animals
Ordinary New Zealand rabbits were provided by Jinan Jinfeng Experimental Animal Co., Ltd.; SPF-grade KM mice were provided by Liaoning Changsheng Biotechnology Co., Ltd.; Hartley guinea pigs were provided by Tengxin Breeding Farm in Bishan District, Chongqing. All animals were raised in the animal breeding room of Chongqing Food and Drug Inspection and Testing Institute, with room temperature of 20~25 ℃, relative humidity of 40%~70%, and 12/12 h alternating light and dark lighting.
1.2 Methods
1.2.1 Preparation of 3D printed porous tantalum sample extract
Use ultrapure water to ultrasonically clean the 3D printed porous tantalum sample for 15 min, dry it naturally, and then sterilize it in an autoclave at 121 ℃ for 30 min for use. According to the provisions of GB/T 16886.12-2017 "Biological Evaluation of Medical Devices Part 12: Sample Preparation and Reference Materials", the cell test uses RPMI. Complete 1640 culture medium as the extraction medium, and the extraction is carried out at 37 ℃ for 24 hours at a ratio of 0.2 g/ml; other test items use 0.9% sodium chloride injection and soybean oil (for injection) as polar solvents and non-polar solvents, respectively, and are added at a ratio of 0.2 g/ml and extracted at 37 ℃ for 72 hours.
1.2.2 Genotoxicity test [8]
According to the recommendations of GB/T 16886.3-2019 "Biological Evaluation of Medical Devices Part 3: Genotoxicity, Carcinogenicity and Reproductive Toxicity Tests", the genotoxicity test is carried out by combining bacterial reverse mutation test, mammalian cell micronucleus test and in vitro mammalian cell chromosome aberration test.
1.2.2.1 Bacterial reverse mutation test
Take 0.1 ml of the frozen solution of qualified TA97, TA98, TA100, TA102 and TA1535 strains and inoculate them into a triangular flask containing 25 ml of nutrient broth medium. Shake and culture until the logarithmic growth phase. Melt the top agar medium and dispense it into sterile test tubes, 2 ml/tube. Keep it warm in a 45 ℃ water bath for later use. Add 0.1 ml of the test strain's new bacterial solution and 0.1 ml of the extract to the warm top agar medium in sequence. Add 0.1 ml of the bacterial solution and 0.1 ml of the extract medium to the solvent control group. If activation is required, add 0.5 ml of S9. After mixing, quickly pour it onto the bottom culture medium. Rotate the plate to evenly distribute the top agar medium on the bottom layer. Lay it flat to solidify. Make 3 plates for each dose. After culturing at 37 ℃ for 48 h, observe and count the number of reverse mutation colonies in each group.
1.2.2.2 Mammalian cell micronucleus test
Fifty healthy adult SPF KM mice, half male and half female, weighing 20-22 g. Randomly divided into polar/non-polar solvent group, polar/non-polar extract group, and positive control group according to body weight, 10 mice in each group, half male and half female. The experiment adopted a 30-hour two-dose method, with each group receiving intraperitoneal injection of 20 ml/kg.bw for two consecutive times with an interval of 24 hours. Bone marrow samples were collected 6 hours after the last dose, mixed with newborn calf serum, and bone marrow smears were prepared, fixed, and stained. Observe and count the number of polychromatic erythrocytes (PCE) and normochromatic erythrocytes (NCE) in 200 erythrocytes under a microscope, and calculate the ratio of PCE/(PCE+NCE); count the number of PCE cells containing micronuclei, and the micronucleus rate is expressed in per thousand.
1.2.2.3 In vitro mammalian cell chromosome aberration test
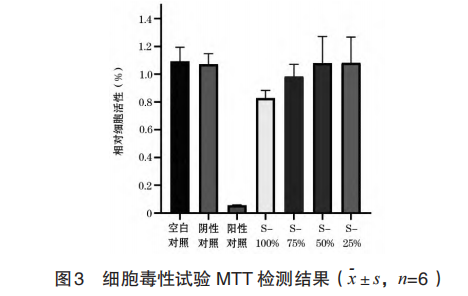
CHL cells that are well attached to the wall in the exponential division phase were taken, and the density was adjusted to 1×106 cells/ml, and cultured for 24 hours. Samples were added according to the sample addition method shown in Table 1, and 2 parallel bottles were set for each group.
4 hours before the termination of the culture, 0.1 ml of 50 μg/ml colchicine was added to 5 ml of the culture medium for each group. At the end of the culture, the cells were collected and counted. After hypotonic treatment, the cell suspension was fixed, sliced, and stained. Chromosome aberrations were observed and recorded under a microscope.
1.2.3 Hemolysis test [9]
Blood was collected from ordinary New Zealand rabbits weighing 2.5 kg to prepare diluted rabbit blood. All test tubes of the polar/non-polar extract group, negative control group, and positive control group were placed in a 37°C water bath for 30 min. 0.2 ml of diluted rabbit blood was added to each test tube, gently mixed, and placed in a 37°C water bath for another 60 min. The liquid in each tube was poured out and centrifuged at 800×g for 5 min. The supernatant was aspirated and the absorbance was measured at a wavelength of 545 nm.
1.2.4 In vitro cytotoxicity test [10]
Take well-growing L929 cells, adjust the density to 1×105 cells/ml, inoculate them in a 96-well plate, and culture for 24 h; aspirate the culture medium, add 100 μl of the test sample 100% extract (S-100%), test sample 75% extract (S-75%), test sample 50% extract (S-50%), test sample 25% extract (S-25%), negative control solution (high-density polyethylene membrane extract), positive control solution (zinc diethyl dithiocarbamate polyurethane membrane extract) and blank culture medium to each well, continue incubation for 24 h, observe the results, and calculate the cell survival rate after MTT test. 1.2.5 Local reaction after implantation [11]
48 normal New Zealand rabbits, half male and half female, weighing 2.5-3.0 kg, were implanted into the muscles of the spinal column parallel to the spine and 25-50 mm from the midline using the rabbit paraspinal muscle implantation method. They were used as the experimental group and the control group. The animals were observed for their general condition after surgery. The animals were painlessly killed on the 14th, 28th, 56th, and 84th day, and gross and histological observations and evaluations were performed.
1.2.6 Intradermal reaction test [12]
3 normal New Zealand rabbits, weighing 2.0-2.5 kg, were injected with polar/non-polar extracts and the corresponding negative control solutions into the hairless area on the side of the spine of each rabbit. The conditions of each injection site were observed and recorded immediately and 24, 48, and 72 hours after injection and scored.
1.2.7 Skin sensitization test [12]
Fifty Hartley guinea pigs, weighing 300-500 g, were randomly divided into a test article polar/non-polar extract group, a polar/non-polar solvent group, and a positive control group, with 10 animals in each group. Induction was performed by intradermal injection of 0.1 ml of the drug into the inner side of the scapula. Seven days after the intradermal injection, a patch was applied to cover the injection site. Fourteen days after the local induction stage, the test article was used for stimulation. The stimulation site of the animals was observed and scored 24 and 48 hours after the end of the test. 1.2.8 Acute systemic toxicity test [13]
20 SPF KM mice, weighing 18-22 g, were randomly divided into the test sample polar/non-polar extract group and polar/non-polar solvent group according to their body weight, with 5 mice in each group; 50 ml/kg.bw was injected intraperitoneally and observed for 72 h after administration.
1.2.9 Subchronic toxicity test [13]
24 ordinary New Zealand rabbits, half male and half female, weighing 2.0-2.5 kg, were randomly divided into the sham operation group and the test sample group according to their body weight, with 12 mice in each group. The test sample in this test was a tantalum disc with a diameter of 10 mm and a thickness of 1 mm. On the first day of the experiment, three tantalum discs were implanted into the dorsal muscles of rabbits; on the 90th day, eight animals were dissected from each group, half of them were female and half were male, and specimens were collected for hematology, blood biochemistry, pathology and other tests; on the 91st day, the implanted tantalum discs in the remaining four animals in the test group were removed, and the animals were allowed to recover for 30 days. On the 121st day, all animals were dissected and specimens were collected for testing.
1.3 Statistical analysis
SPSS 19.0 statistical software was used for data analysis, and the quantitative data were expressed as x-±s. One-way analysis of variance was used, and P<0.05 was considered statistically significant.
2 Results
2.1 Genetic toxicity test
2.1.1 Bacterial reverse mutation test
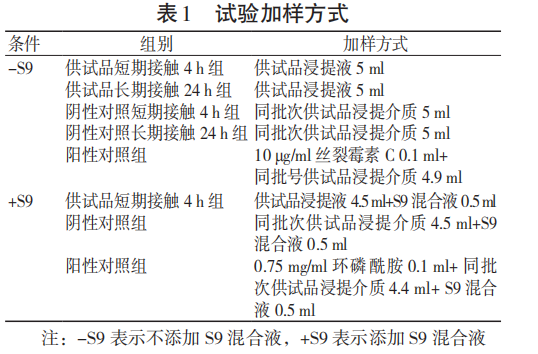
As shown in Figure 1, the number of reverse mutation colonies of TA97, TA98, TA100, and TA102 strains in the polar/non-polar extract group of the test sample with and without the metabolic activation system was less than 2 times the count of the solvent control group, and the number of reverse mutation colonies of TA1535 strain was less than 3 times the count of the solvent control group, indicating that the 3D printed porous tantalum bacterial reverse mutation test was negative.
2.1.2 Mammalian cell micronucleus test
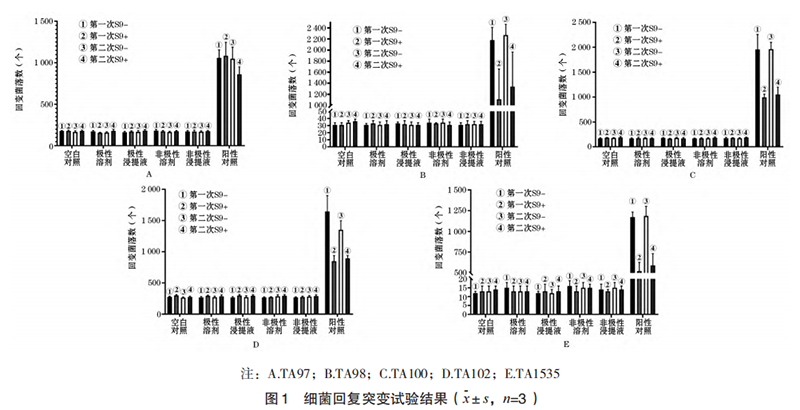
2.1.2.1 PCE/(PCE+NCE) ratio As shown in Figure 2A, the PCE/(PCE+NCE) ratio of the bone marrow of mice in the polar/non-polar extract group of the test sample was not statistically significant compared with the corresponding solvent control group (P>0.05), indicating that the 3D printed porous tantalum sample had no in vivo bone marrow cytotoxicity.
2.1.2.2 Results of micronucleus test
As shown in Figure 2B, the number of micronuclei in PCE of mice in the positive control group increased significantly. Compared with the solvent control group and the polar/non-polar extract group of the test sample, the micronucleus rate increased significantly, and the difference was statistically significant (P<0.01), indicating that the test was established; the PCE micronucleus rate of mice in the polar/non-polar extract group of the test sample was compared with the corresponding solvent control group, and there was no statistically significant difference (P>0.05), indicating that 3D printed porous tantalum has no in vivo genetic toxicity.
2.1.3 Chromosome aberration test of mammalian cells in vitro
Toxicity of 3D printed porous tantalum extract on CHL cells: Cytotoxicity results showed that the relative cell growth of 3D printed porous tantalum extract was 84.68%, 85.07% and 84.68% respectively when it was exposed to CHL cells for 4 h with or without S9, and for 24 h without S9, all of which were greater than 80%, indicating no cytotoxicity.
Effect of 3D printed porous tantalum extract on CHL cell chromosomes: Chromosome aberration observation results showed that no aberrant cells were found when 3D printed porous tantalum extract was exposed to CHL cells for 4 h with or without S9, and for 24 h without S9. The difference in chromosome aberration rate was not statistically significant compared with the negative control group (P>0.05), indicating that 3D printed porous tantalum had no chromosomal aberration effect.
The results of the bacterial reverse mutation test, mammalian cell micronucleus test and in vitro mammalian cell chromosome aberration test indicate that 3D printed porous tantalum has no genetic toxicity.
2.2 Hemolysis test
The hemolysis rates of the polar/non-polar extracts of the test sample were 3.24% and 0%, respectively, both lower than the 5% specified in the standard, indicating that 3D printed porous tantalum has no hemolysis reaction.
2.3 CellToxicity test
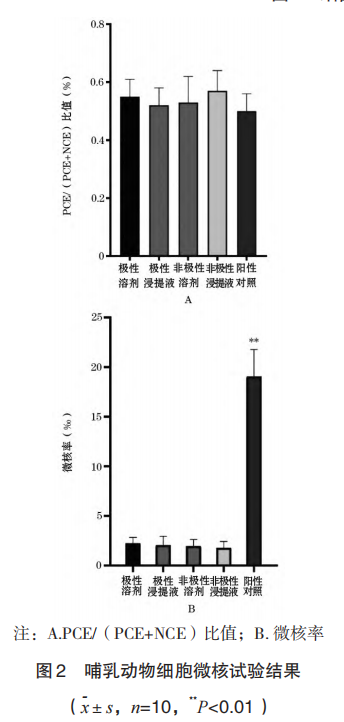
From the perspective of cell morphology, the cells in the 3D printed porous tantalum extract group grew well, and there was no difference in cell morphology from the blank control group; in the MTT test, the blank control group was taken as 100% for survival rate calculation, and the 3D printed porous tantalum extract solution (S-100%) and three concentration dilutions (S-75%, S-50%, S-25%) were in contact with L929 cells for 24 hours. The cell survival rate was greater than 80%, which was greater than 70% of the blank control group. The survival rate of the S-50% group was greater than that of the S-100% group. The specific results are shown in Figure 3, indicating that 3D printed porous tantalum has no potential cytotoxicity.
2.4 Local reaction after implantation
During the experiment, all experimental animals were in good general condition after surgery, eating and moving normally, there was no swelling or secretion in the wound, no infection occurred, and no material was discharged. The wounds healed in 2 weeks.
During the autopsy, the naked eye observation showed that the implantation position of the tantalum and titanium discs was basically normal, the fibrous capsule was well formed, the tantalum and titanium discs were tightly combined with the surrounding muscles, without displacement or dislocation, and the removal was smooth. There was no obvious adhesion between the fibrous tissue of the capsule wall and the tantalum and titanium discs; the size of the implanted material was measured when the material was taken, and the appearance size remained unchanged compared with that before the operation.
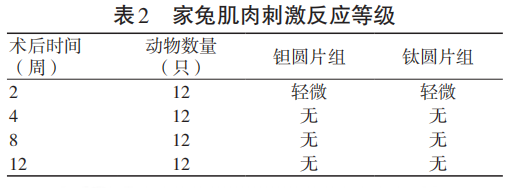
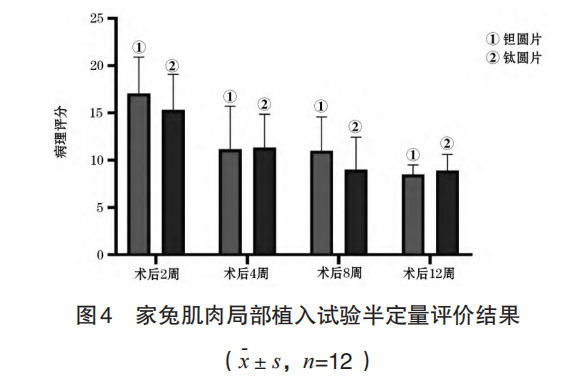
The muscle stimulation response results of the local implantation test in rabbit muscles are shown in Table 2, and the pathological semi-quantitative evaluation results are shown in Figure 4. The test results showed that there was no statistically significant difference between the muscle stimulation response level at each observation time point after surgery and the local tissue pathological change score after implantation in the test group and the control group (P>0.05).
2.5 Intradermal reaction test
The scores of the test polar/non-polar extract group and the negative control group were all 0 at 24, 48, and 72 hours after injection, and no stimulation reaction was observed. The test results showed that 3D printed porous tantalum had no intradermal irritation effect.
2.6 Skin sensitization test
The scores of the polar/non-polar extract group and the polar/non-polar solvent group of the test sample were 0 at 24 and 48 hours after stimulation, and no skin sensitization reaction was observed; in the positive control group, 1 animal scored 2 points and 2 animals scored 1 point 24 hours after removing the patch; 2 animals scored 1 point at 48 hours, and skin sensitization occurred. The test results show that repeated contact with 3D printed porous tantalum does not cause sensitization reaction in Hartley guinea pigs.
2.7 Acute systemic toxicity test Intraperitoneally injected with polar/non-polar extract of 3D printed porous tantalum, mice showed no poisoning symptoms or adverse reactions during the 72-hour observation period, and the body weight of mice increased normally. The test results show that 3D printed porous tantalum does not cause acute systemic toxicity.
2.8 Subchronic toxicity test
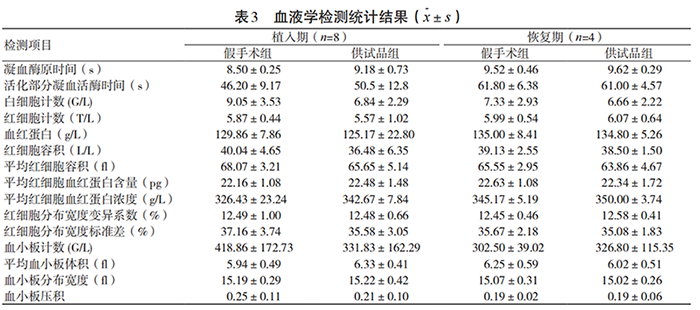
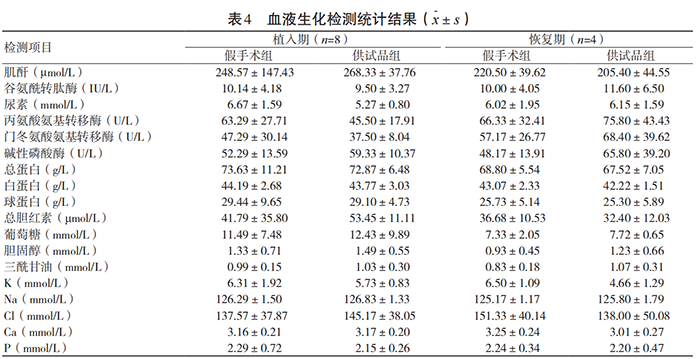
During the test, all experimental animals were in good general condition after surgery, and their eating and activities were normal. There were no statistically significant differences in the weight growth rate, food intake, main organ weight ratio and urine routine of the test group animals during the implantation and recovery periods compared with the sham operation group (P>0.05). There were no statistically significant differences in 15 hematological indicators and 18 blood biochemical indicators between the test group and the sham operation group (P>0.05). The specific results are shown in Tables 3~4. Pathological morphological examinations were performed on the liver, heart, spleen, lung, kidney, brain and other tissues, organs and test site muscles of the test group and sham operation group animals. No obvious toxic effects were found in the tissues, organs and test site muscles.
3 Discussion
Porous tantalum is a new generation of ideal implant materials. With the development of personalized medicine, 3D printing of porous tantalum has become a research hotspot. At present, there are many studies on the preparation process and clinical treatment effectiveness of 3D printed porous tantalum [14]. Most of the studies on safety are in vitro cell tests, and there are few studies on in vivo safety. Therefore, the biosafety of 3D printed porous tantalum needs more comprehensive and systematic test data to confirm. This study conducted a comprehensive biosafety evaluation of 3D printed porous tantalum materials for surgical implants based on the requirements for implant materials in the GB/T 16886 "Biological Evaluation of Medical Devices" standard. The test results show that 3D printed porous tantalum has no genotoxicity, does not cause hemolytic reaction, has no potential cytotoxicity, does not cause intradermal irritation, has no skin sensitization, does not cause acute systemic toxicity, and has no subchronic systemic toxicity, which meets the national standards for clinical application of medical materials.
The local reaction and subchronic toxicity test of long-term implant products after implantation should be the focus of safety evaluation. Compared with the rat tail vein injection method for subchronic systemic toxicity investigation, this study used the New Zealand rabbit muscle implantation method to reproduce the clinical use of the material and more directly and truly reflect the safety of the implant material product; at the same time, titanium [15], a material widely used in clinical practice and with relatively mature biocompatibility research, was used as the reference sample for the local reaction test after implantation. The test results showed that there was no significant difference in all indicators between the test sample and the reference sample.
In short, 3D printed porous tantalum is a promising material in the biomedical field with good biocompatibility. This study provides a scientific basis for the safe application of 3D printed porous tantalum in the field of surgical implantation.
Paper citation information
Medical Equipment May 2022 Vol. 35 No. 9
Medical Grade Spherical Tantalum Powder
The spherical tantalum powder produced by Stardust Technology using radio frequency plasma spheroidization technology has excellent biocompatibility and bone ingrowth characteristics, and is widely used in 3D printing of clinical medical implants such as spine, joints, and trauma. Stardust Technology is the first in China to achieve an industrial breakthrough in medical-grade spherical tantalum powder. It has jointly promoted the clinical medical application of tantalum metal with domestic first-class orthopedic hospitals and medical device companies. It has participated in the formulation of GB/T 38975-2020 Tantalum and tantalum alloy powder for additive manufacturing, GB/T 41883-2022 Powder bed fusion additive manufacturing of tantalum and composite alloys related national standards, YY/T1851-2022 Medical pure tantalum powder for additive manufacturing industry standard 1, T/CAMDI065-2021 Additive manufacturing tantalum metal knee prosthesis, T/CAMDI 066-2021 Additive manufacturing tantalum metal individualized bone defect filler "and other group standards, and assisted in the completion of more than 500 cases of tantalum metal clinical application. Implanted prostheses include tantalum metal intervertebral fusion bone spacers, hip joints, shoulder joints, knee joints, ankle joints, etc.
https://www.stardustpowder.com/tantalum-1

For more details, please contact Vicky at +86-13318326185
