
Tantalum is a kind of refractory metal with high melting point and high boiling point. It has excellent processing performance and corrosion resistance [1] and is mainly used as capacitors, rare earth smelting and corrosion-resistant containers for chemical industry [2-3]. Compared with pure tantalum, tantalum carbide has higher strength and hardness [4-6], excellent ablation resistance and wear resistance, and is currently mainly used in mechanical processing, aerospace and other fields [7-11]. The disadvantage of tantalum carbide is that its processing performance at room temperature is poor and it is not easy to form, which limits its application range. Existing studies [12] have shown that coating tantalum carbide on the surface of metallic tantalum can protect the matrix structure in a corrosive environment, improve its oxidation resistance and chemical inertness, and at the same time, the chemical stability and hardness of the carburized layer increase with the increase of the ratio of TaC and Ta2C [13-15]. In industry, carburizing, chemical deposition, plasma spraying and other methods are generally used to obtain tantalum carbide layer on the tantalum surface [16-17]. Carburizing is the most widely used chemical heat treatment method in the machinery manufacturing industry. It involves infiltrating active carbon atoms into the surface of a workpiece, increasing the surface carbon concentration and thereby obtaining a high-carbon carburized layer structure [18-19]. Currently, the main carburizing methods include gas carburizing, solid carburizing, vacuum carburizing, and ion carburizing. Compared with traditional carburizing methods, vacuum carburizing has the advantage of good carburizing uniformity and significantly reduces carbon dioxide and harmful chemical emissions, making it more environmentally friendly. Currently, carburizing treatment of tantalum and its alloys generally uses gas carburizing to attach a layer of carbide to the surface to improve the strength, hardness, and ablation resistance of the workpiece. The disadvantage is that the gas carburizing process is prone to produce intergranular oxides, which reduces the reliability and service life of the workpiece. The use of vacuum carburizing technology can avoid the generation of oxides and ensure the quality of the workpiece after carburization. Currently, there are no reports on the use of vacuum carburizing technology for surface treatment of Ta and Ta-W alloys, and the influence of specific process parameters on the phase composition and thickness of the carburized layer remains to be studied. This study examined the effects of carburizing time and temperature on carburizing results using Ta, Ta-2.5% W, and Ta-7.5% W as experimental samples. The influence of alloying element W on the carburizing results of tantalum alloys was also investigated.
1 Experiment
Ta, Ta-2.5% W, and Ta-7.5% W were carburized using vacuum carburizing. Acetylene was used as the infiltration gas, with a carburizing-to-load ratio of 1:8. The carburizing temperature was 1300-1500°C, and the holding time was 5-10 hours. The samples were then cooled in the furnace after carburizing. A modified vacuum carbon tube furnace was used for carburizing. Before carburizing, the samples were surface treated to remove impurities, oil, and scale, while maintaining a consistent surface roughness to eliminate interference from other factors.
2 Results and Discussion
2.1 Phase Analysis of the Carburized Layer
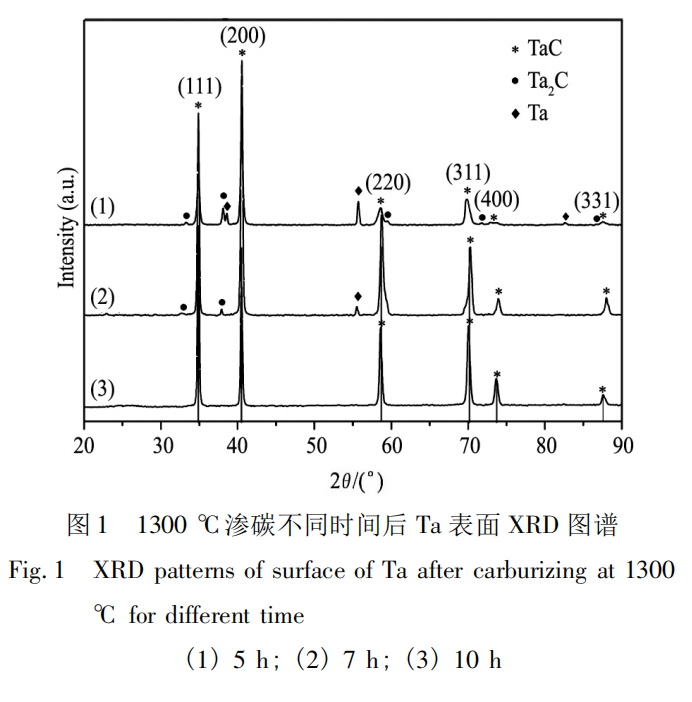
Pure Ta was carburized at 1300°C for different times. The phase composition of the carburized layer is shown in Figure 1. Analysis shows that after 5 hours of carburization, the carburized layer of pure Ta is composed of three phases: Ta, TaC, and Ta2C. X-ray diffraction (XRD) analysis after 7 hours of carburization reveals that with increasing carburization time, the Ta and Ta2C phases gradually decrease, while the TaC phase gradually increases. Extending the carburization time to 10 hours results in the formation of TaC entirely on the surface of the carburized layer. The Ta-C carburization reaction is a process of carbon diffusion into Ta. Initially, carbon atoms diffuse into the Ta and fill the octahedral interstices within the crystal. As diffusion proceeds, the concentration of carbon atoms in the lattice interstices increases. When this concentration reaches saturation, a Ta2C interstitial phase with a hexagonal close-packed (hcp) structure begins to form. The following chemical reactions mainly occur during this process:

At this time, C only fills part of the octahedral gaps. As the carburizing time increases, the excess C occupies the octahedral gaps of the Ta2C lattice, and the crystal structure begins to transform (hcp→fcc), starting to form TaC with a cubic (fcc) structure. At the same time, the unreacted Ta and C continue to generate Ta2C. Formula (2) is the main chemical reaction in this stage:
As carburizing continues, the Ta phase disappears and forms Ta2C. When the C atoms fill the octahedral gaps, TaC is finally generated. X-ray diffraction (XRD) patterns show that under this experimental condition, the phase composition of the carburized layer is closely related to the carburizing time. When the carburizing time is short, the sample surface cannot fully react with the C-containing atmosphere, resulting in the presence of unreacted Ta and intermediate phase Ta2C on the surface after the carburizing is completed; extending the carburizing time will eventually result in the generation of TaC.
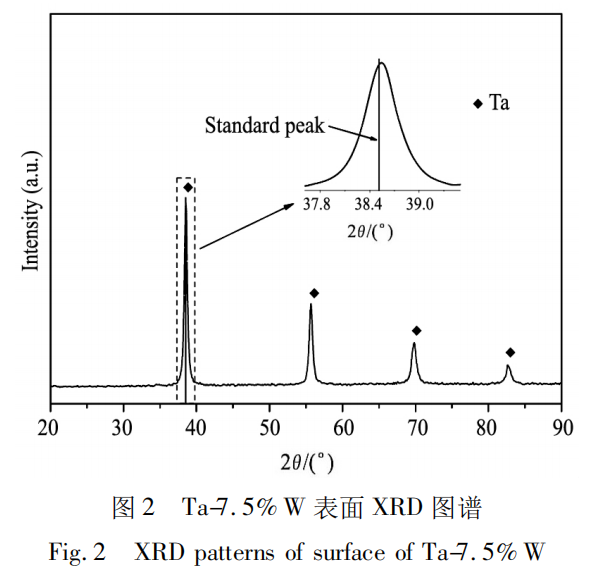
As shown in Figure 2, the surface of the Ta-7.5% W alloy before carburization consists of a single Ta phase. The Ta-W phase diagram shows that the Ta-W alloy is an infinite substitutional solid solution. W dissolution only replaces some of the solvent atoms, but the crystal structure remains unchanged, maintaining the original body-centered cubic structure. Therefore, the XRD pattern indicates that the alloy consists solely of the Ta phase. The difference is that the XRD pattern in Figure 2 exhibits a slight offset from the standard peak for Ta. This is because W atoms are slightly smaller than Ta atoms, and their dissolution reduces the Ta lattice constant, resulting in a deviation in the peak position from the standard peak on the PDF card.
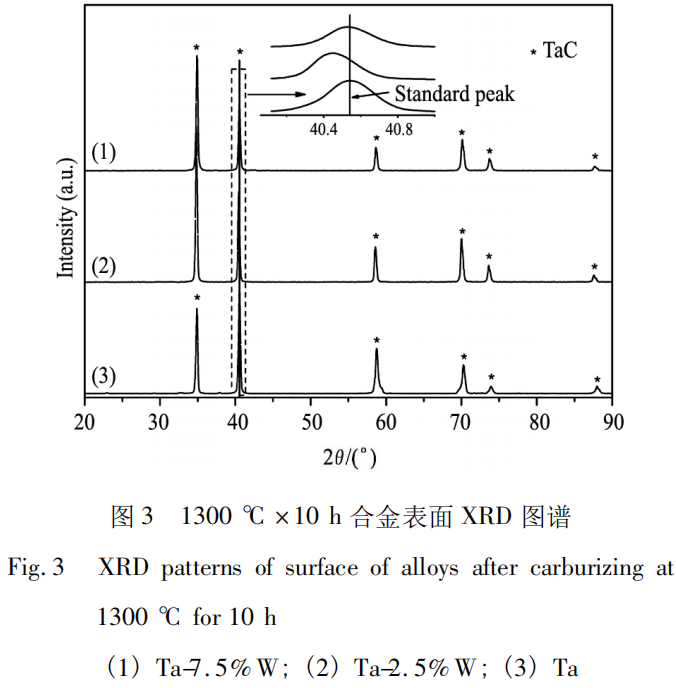
 Phase analysis of Ta, Ta-2.5% W, and Ta-7.5% W after carburization at 1300°C for 10 hours is shown in Figure 3. Analysis indicates that under these experimental conditions, TaC formed on the surface of all samples, and no W-containing phase was detected. Composition analysis using energy dispersive spectroscopy (EDS) revealed the presence of W in the carburized layer. This indicates that under these experimental conditions, carbon atoms reacted only with Ta to form tantalum-carbon compounds, while W remained dissolved as a solute atom within the crystal. Compared to pure Ta, the XRD patterns of Ta-2.5%W and Ta-7.5%W were affected by the presence of W, resulting in a shift in their XRD patterns.
Phase analysis of Ta, Ta-2.5% W, and Ta-7.5% W after carburization at 1300°C for 10 hours is shown in Figure 3. Analysis indicates that under these experimental conditions, TaC formed on the surface of all samples, and no W-containing phase was detected. Composition analysis using energy dispersive spectroscopy (EDS) revealed the presence of W in the carburized layer. This indicates that under these experimental conditions, carbon atoms reacted only with Ta to form tantalum-carbon compounds, while W remained dissolved as a solute atom within the crystal. Compared to pure Ta, the XRD patterns of Ta-2.5%W and Ta-7.5%W were affected by the presence of W, resulting in a shift in their XRD patterns.
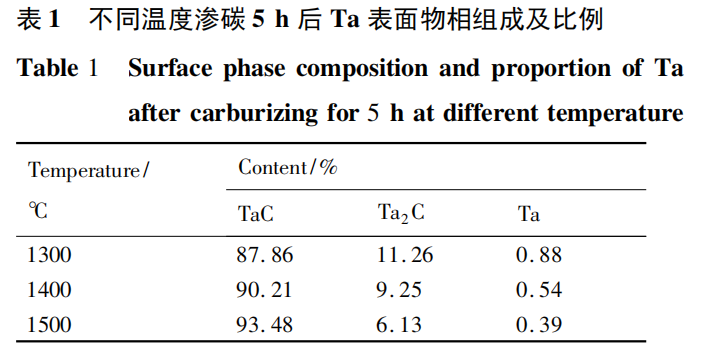
The phase composition and proportion of Ta surfaces after carburizing for 5 h at different temperatures were analyzed, as shown in Table 1. The analysis revealed that after carburizing for 5 h at 1300°C, the TaC content on the Ta surface was 87.86%, 90.21% at 1400°C, and 93.48% at 1500°C. This indicates that, for the same carburizing time, the proportion of TaC formed on the surface increases with increasing temperature. This is because higher temperatures increase the activity of carbon atoms, facilitating diffusion reactions and, to some extent, promoting the formation of TaC.
2.2 Effect of Carburizing Process on Carburizing Results
To investigate the effect of vacuum carburizing on the carburized layer thickness of Ta and Ta-W alloys, pure Ta, Ta-2.5%W, and Ta-7.5%W were carburized at different times and temperatures. The experimental temperatures were 1300, 1400, and 1500°C, and the carburizing times were 5.7 and 10 hours. As shown in Figure 4, the carburized samples exhibited a uniform yellow color (tantalum carbide is yellow) and good gloss.

2.2.1 Effect of Carburizing Temperature on Carburized Layer Thickness
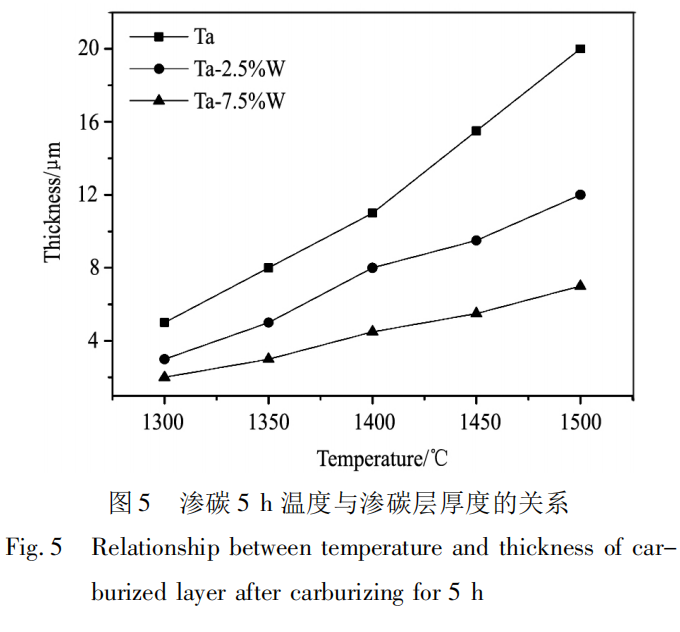
At lower carburizing temperatures, the carburized layer thickness is smaller. Therefore, the carburized layer thickness was measured at five different locations on each sample, and the average value was used as the actual carburized layer thickness, with an error of 0.5 to 1.0 μm. The samples were carburized for 5 hours at temperatures between 1300 and 1500°C. The experimental results are shown in Figure 5. Analysis shows that increasing the carburizing temperature significantly increases the thickness of the carburized layer. From a thermodynamic perspective, the main factors affecting diffusion are temperature and diffusion activation energy, and their relationship with the diffusion coefficient is shown in formula (3):

In the formula, D0 is the frequency factor, Q is the diffusion activation energy, R is the gas constant, and T is the temperature. According to the formula, increasing the reaction temperature or reducing the diffusion activation energy can effectively increase the diffusion coefficient, thereby reducing the diffusion difficulty of C atoms. On the one hand, increasing the carburizing temperature can accelerate the decomposition of acetylene and increase the activity of C atoms, thereby greatly improving the efficiency of the diffusion reaction between C atoms and the matrix. On the other hand, as the carburizing temperature increases, the density of defects such as vacancies in the Ta matrix increases, which increases the diffusion rate of C atoms in the matrix. At the same time, the diffusion rate of Ta atoms in the alloy also increases significantly, and the generation rate of the carburized layer is accelerated. Therefore, a thicker carburized layer can be obtained after carburizing.
2.2.2 Effect of carburizing time on carburized layer thickness
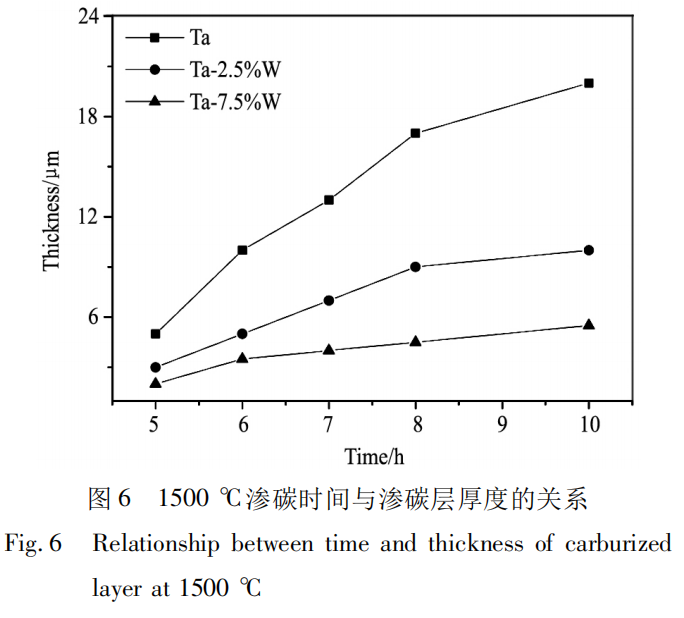
Figure 6 is a curve showing the relationship between carburized layer thickness and time after carburizing at 1500℃. It can be observed that the carburized layer thickness increases with the extension of carburizing time. Reference [21] points out that the carburized layer thickness mainly depends on time and temperature, and the relationship with time satisfies the parabolic form:

Wherein, W is the carburized layer thickness, K is a function of carburizing temperature and activation energy, and t is time. That is, at the same carburizing temperature, the carburized layer thickness increases with the extension of carburizing time, and the carburized layer growth rate slows down. According to Fick's first law, the magnitude of the diffusion flux is proportional to the concentration gradient:

Wherein, J is the diffusion flux, D is the diffusion coefficient, and dc/dx is the concentration gradient. The direction of diffusion is opposite to the positive direction of the concentration gradient, so the macroscopic flow of diffusion always proceeds from high solute concentration to low solute concentration. Therefore, the diffusion rate of C atoms is affected by the concentration gradient. The larger the concentration gradient, the faster the diffusion rate. In the early stages of carburization, the concentration difference between the inside and outside of the sample is large, leading to a rapid diffusion rate of carbon atoms. Over time, some carbon atoms diffuse into the matrix, reducing the concentration difference. Furthermore, TaC forms on the sample surface, transforming the crystal structure from body-centered cubic to face-centered cubic. This reduces the lattice interstices and increases the activation energy required for carbon atom transitions. Therefore, the carburized layer thickness increases with time during carburization, but the carbon atom diffusion rate decreases, slowing the rate of carburized layer growth.
2.3 Effect of Alloying Element W on Carburization Results of Ta-W Alloys
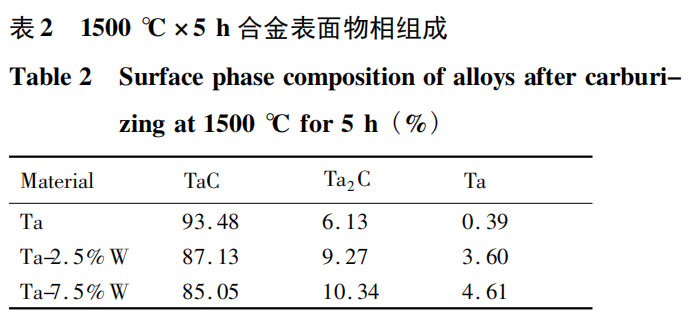
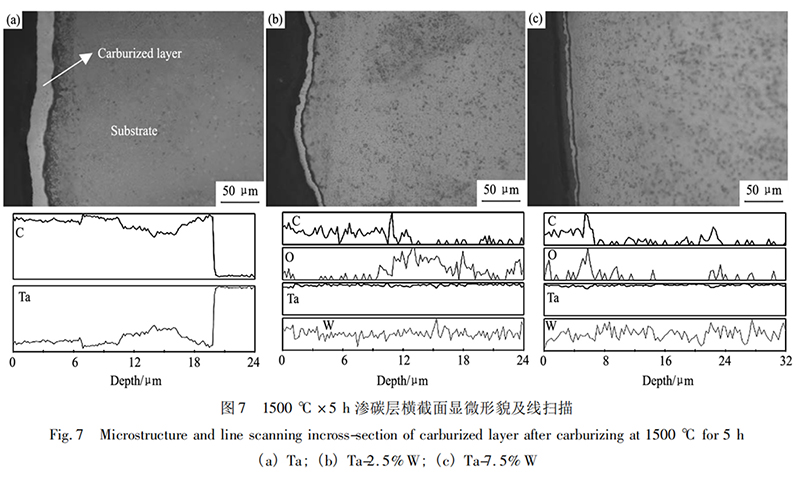
Figure 7 shows the cross-sectional micromorphologies of Ta, Ta-2.5%W, and Ta-7.5%W after carburization at 1500℃ for 5 hours. The corresponding line scan curves are shown below. Analysis shows that under these experimental conditions, the carburized layer thickness of Ta metal is approximately 20 μm, the carburized layer thickness of Ta-2.5%W is approximately 10-12 μm, and the carburized layer thickness of Ta-7.5%W is only 7-8 μm. The results show that the higher the W content in the alloy, the thinner the carburized layer obtained after carburization. This phenomenon suggests that W hinders the carburization process of tantalum-tungsten alloys. During carburization, carbon atoms exist as interstitial solutions in the lattice interstices. Over time, tantalum-carbon compounds are formed. Adding W to Ta forms a substitutional solid solution. The smaller atomic radius of the solute W atoms causes the surrounding lattice to contract, reducing the average lattice constant and distorting the lattice, resulting in changes in the lattice interstices within the crystal structure. This phenomenon reduces the solubility of carbon atoms in the Ta alloy and significantly increases the activation energy required for carbon atoms to diffuse into the Ta matrix. Therefore, the presence of W in the Ta matrix hinders the diffusion of carbon atoms. As the W content increases, carburization becomes more difficult and the resulting carburized layer becomes thinner. The surface phase composition and proportions of Ta, Ta-2.5% W, and Ta-7.5% W after carburizing at 1500℃ for 5 h were analyzed. The results are shown in Table 2. The ratio of TaC to Ta2C in the carburized layer decreases with increasing W content, further demonstrating that the presence of W in tantalum-tungsten alloys hinders the diffusion reaction. When the carburizing time is extended to 10 h, TaC is completely formed on the surface. This indicates that within these experimental conditions, the alloying element W slows the carburizing reaction. Prolonging the carburizing time allows the tantalum-tungsten alloy surface to fully react with the C-containing atmosphere, ultimately forming TaC.
3. Conclusion
1. When tantalum metal is carburized at 1300℃, the surface phases are primarily TaC and Ta2C. After 5 h of carburization, TaC, Ta2C, and Ta coexist on the surface. With increasing carburizing time, the Ta and Ta2C phases disappear sequentially, and after 10 h of carburization, TaC is completely formed on the surface.
2. Increasing the carburizing temperature within the range of 1300-1500°C increases the carburized layer thickness. Prolonging the carburizing time increases the carburized layer thickness, but the rate of increase decreases. Carburizing for 10 hours at 1500°C results in a maximum carburized layer thickness of 35-40 μm for tantalum metal.
3. Elemental W inhibits the carburizing process of tantalum-tungsten alloys. Under the same carburizing conditions, a higher W content in the matrix results in a slower carburizing rate and a smaller carburized layer thickness.
Paper Citation Information
Rare Metals Volume 42, Issue 9 September 2018
Medical-Grade Spherical Tantalum Powder
Spherical tantalum powder produced by Stardust Technology using radio frequency plasma spheroidization technology boasts excellent biocompatibility and bone ingrowth properties, making it widely used in 3D printing of clinical medical implants for the spine, joints, and trauma applications. Stardust Technology pioneered the industrialization of medical-grade spherical tantalum powder in China. Working with leading domestic orthopedic hospitals and medical device companies, Stardust Technology has promoted the clinical application of tantalum metal. The company has participated in the development of national standards, including GB/T 38975-2020 Tantalum and Tantalum Alloy Powders for Additive Manufacturing, GB/T 41883-2022 Powder Bed Fusion Additive Manufacturing of Tantalum and Composite Alloys, YY/T1851-2022, an industry standard for medical pure tantalum powder for additive manufacturing, and three group standards, including T/CAMDI 065-2021 Additive Manufacturing of Tantalum Knee Prostheses and T/CAMDI 066-2021 Additive Manufacturing of Tantalum Personalized Bone Defect Fillers. Stardust Technology has assisted in the clinical application of tantalum metal in over 500 cases, including implants for tantalum intervertebral fusion devices, bone spacers, hip, shoulder, knee, and ankle joints.
https://www.stardustpowder.com/spherical-pure-ta-powder
For more information on spherical tantalum powder and tantalum alloy powders, please contact Vicky Zhang +86-13318326185
